General Chemistry
Solutions to Over 1000 Practice Questions
Chemistry and Math
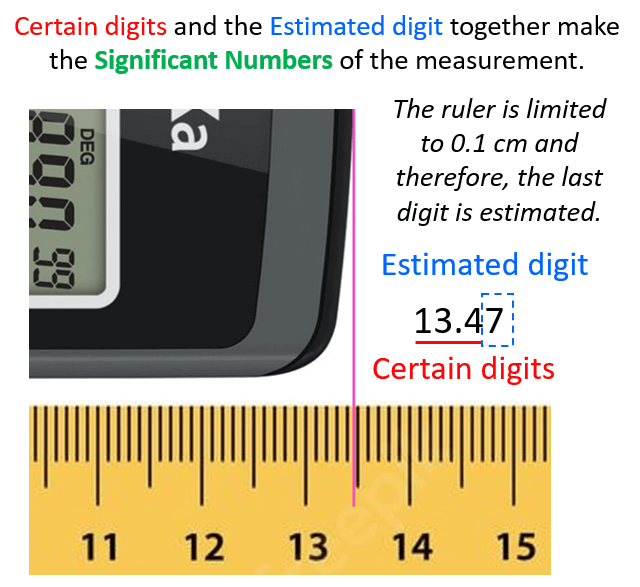
- Significant Figures
- Significant Figures in Addition, Subtraction Multiplication, and Division
- Significant Figures Practice Problems
- Converting Units With Conversion Factors Dimensional Analysis
- Conversion Factors and Dimensional Analysis Practice Problems
- Density Practice Problems
The Components of Matter
- Pure Substances, Mixtures, Elements, and Compounds
- Heterogeneous and Homogeneous Mixtures
- States of Matter: Solid, Liquid, Gas, and Plasma
Atoms, Molecules, and Ions
- Subatomic particles and Isotopes
- What are Isotopes?
- How To Calculate The Number of Protons, Neutrons, and Electrons
- The Average Atomic Mass
- Calculating The Percent Abundance of Each Isotope
- Naming Monatomic and Polyatomic Ions
- Naming Ionic Compounds
- Writing Chemical Formulas For Ionic Compounds
- Naming Covalent Compounds
- Naming Acids and Bases
- Atomic and Molecular Masses
Stoichiometry
- The Mole and Molar Mass
- How To Calculate The Molar Mass
- How To Convert Grams To Moles
- Grams to Molecules and Molecules to Mass
- How To Convert Grams To Number of Atoms
- Mass, Moles, and Number of Particles Practice
- Percent Composition and Empirical Formula
- Percent Composition and Empirical Formula Practice Problems
- Stoichiometry of Chemical Reactions
- Limiting Reactant
- How To Find The Amount of Excess Reactant
- Limiting Reactant Practice Problems
- Reaction/Percent Yield
- Stoichiometry Practice Problems
- Mass, Moles, and Number of Particles Quiz
- The Stoichiometry of Chemical Reactions Quiz
Reactions in Aqueous Solutions
- Solutions
- Strong and Weak Electrolytes
- Dissociation of Ionic Compounds
- Molecular, Ionic, and Net Ionic Equations
- Molarity
- Dilution
- Ion Concentration
- Precipitation Reactions
- Definitions of Acids and Bases
- Naming Acids and Bases
- Acid-Base Reactions
- Displacement Reactions
- Predicting The Products of Chemical Reactions
- Stoichiometry of Reactions in Aqueous Solutions
- Acid-Base Titrations
- Oxidation State
- Oxidation-Reduction (Redox) Reactions
- Oxidizing and Reducing Agents
- Balancing Redox Reactions
- Oxidation State Practice Problems
- Reactions in Aqueous Solutions – Practice Problems
Reactions in Aqueous Solutions Quiz
Gases
- Boyle’s Law
- Charle’s Law
- Gay-Lussac’s Law
- Avogadro’s Law
- The Ideal Gas Law
- Celsius or Kelvin
- Ideal-Gas Laws
- Combined Gas Law Equation
- How to Know Which Gas Law Equation to Use
- Molar Mass and Density of Gases
- Graham’s Law of Effusion and Diffusion
- Graham’s Law of Effusion Practice Problems
- Dalton’s Law of Partial Pressures
- Mole Fraction and Partial Pressure of the Gas
- Gases in Chemical Reactions
- Gases-Practice Problems
- A Multiple-Choice Quiz on Gases
Thermochemistry
- Energy Related to Heat and Work
- Endothermic and Exothermic Processes
- Heat Capacity and Specific Heat
- Heat Capacity Practice Problems
- Heat and Phase Change Diagrams
- What is Enthalpy
- Constant-Pressure Calorimetry
- Bomb calorimeter – Constant Volume Calorimetry
- Calorimetry Practice Problems
- Stoichiometry and Enthalpy of Chemical Reactions
- Hess’s Law and Enthalpy of Reaction
- Hess’s Law Practice Problems
- Standard Enthalpies of Formation
- Enthalpy of Reaction from Enthalpies of Formation
- Thermochemistry Practice Problems
- Thermochemistry Quiz
Electronic Structure of Atoms
- Atomic Orbitals
- Electron Configurations
- Electron Configurations of Ions
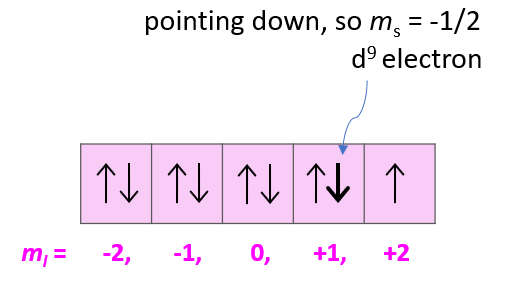
- Orbital Diagrams
- Aufbau’s Principle, Hund’s Rule, and Pauli’s Exclusion Principle
- Hund’s Rule
- Pauli Exclusion Principle
- Quantum Numbers (n, l, ml, ms)
- Bohr Model of the Hydrogen Atom
- Rydberg Formula
- The Photoelectric Effect
- Calculating The Energy of a Photon
- Effective Nuclear Charge
- Atomic Radius
- Ionic Radius
- Ionization Energy
- Electron Affinity
- Energy, Wavelength, and Frequency Practice Problems
- Electronic Structure of Atoms Quiz
- Periodic Table and Periodic Trends
Concepts of Chemical Bonding
- Lewis Dot Symbols
- Valence Electrons
- The Ionic Bond
- The Covalent Bond
- Sigma and Pi Bonds
- Electronegativity and Bond Polarity
- The Octet Rule
- Formal Charges
- Lewis Structures and the Octet Rule
- Lewis Structures Practice Problems
- Resonance Structures
- Chemical Bonding and Lewis Structures Quiz
Molecular Geometry
Intermolecular Forces in Liquids and Solids
Properties of Solutions
- Molality
- Colligative Properties
- Vapor Pressure Lowering
- Boiling Point Elevation
- Freezing Point Depression
- Osmotic Pressure
- Molarity, Molality, and Other Concentrations – Practice Problems
- Colligative Properties Practice Problems
Chemical Kinetics
- Reaction Rate
- Rate Law and Reaction Order
- How to Determine the Reaction Order
- Integrated Rate Law
- The Half-Life of a Reaction
- Half-Life and Radioactivity Practice Problems
- First-Order Reactions
- Second-Order Reactions
- Zero-Order Reactions
- Determining the Reaction Order Using Graphs
- Units of Rate Constant k
- How Are Integrated Rate Laws Obtained
- Activation Energy
- The Arrhenius Equation
- Chemical Kinetics Practice Problems
- Chemical Kinetics Quiz
Chemical Equilibrium
- Chemical Equilibrium
- Equilibrium Constant
- Kp and Partial Pressure
- Kp and KcRelationship
- K Changes with Chemical Equation
- Equilibrium Constant K from Two Reactions
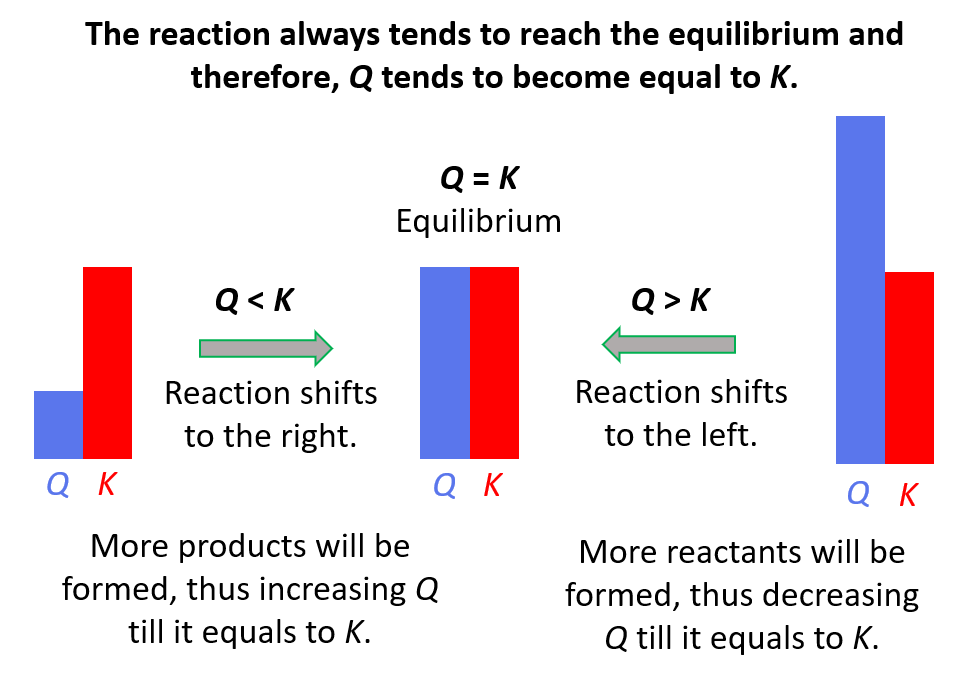
- Reaction Quotient – Q
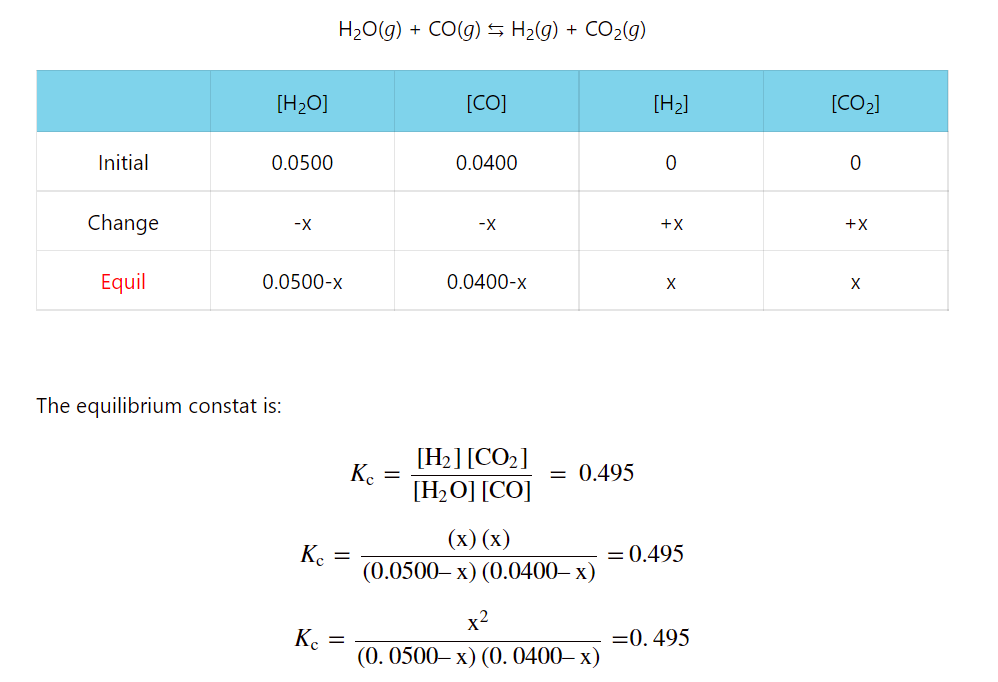
- ICE Table – Calculating Equilibrium Concentrations
- ICE Table Practice Problems
- Le Châtelier’s principle
- Le Châtelier’s Principle Practice Problems
- Chemical Equilibrium Practice Problems
- Chemical Equilibrium Quiz
Acids and Bases
- Definitions of Acids and Bases
- Acid-Base Reactions
- Acid-Base Titrations
- Conjugate Acid and Conjugate Base
- Autoionization of Water and Kw
- The pH and Acidity
- Acid Strength, Ka, and pKa
- Base Strength, Kb, and pKb
- Ka, pKa, Kb, and pKb Relationship
- The pH of a Strong Acid and Base
- pH + pOH = 14
- The pH of a Weak Acid
- The pH of a Weak Base
- The pH of Polyprotic Acids
- The acidity of a Salt Solution
- The pH of a Salt Solution
- The pH of Salts With Acidic Cations and Basic Anions
- pH Practice Problems
- Acids and Bases Practice Problems
Acids and Bases Quiz
Acid-Base and Solubility Equilibria
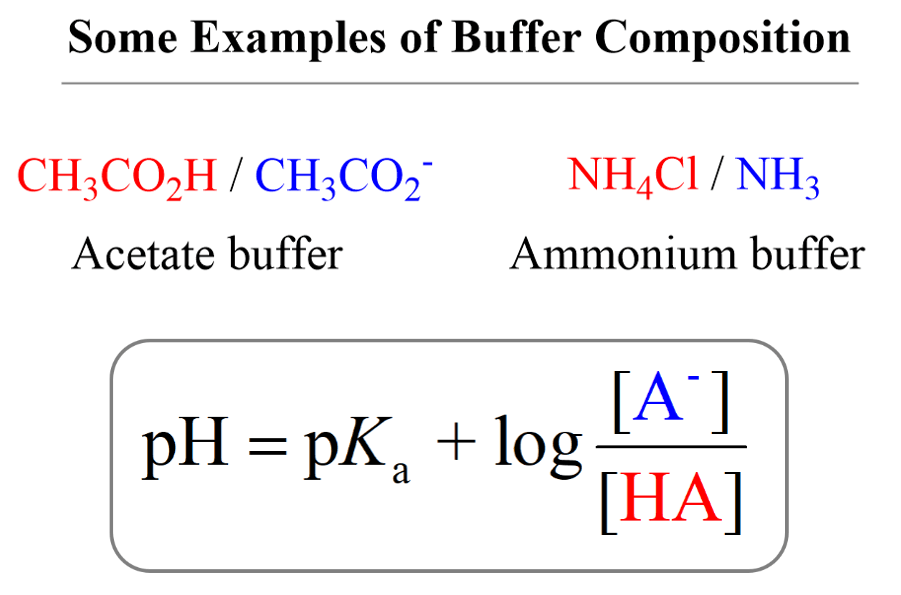
- Buffer Solutions
- The Henderson–Hasselbalch Equation
- The pH of a Buffer Solution
- Preparing a Buffer with a Specific pH
- The Common Ion Effect
- The pH and pKa Relationship
- Strong Acid–Strong Base Titrations
- Titration of a Weak Acid by a Strong Base
- Titration of a Weak Base by a Strong Acid
- Titration of Polyprotic Acids
- Buffer Solutions Practice Problems
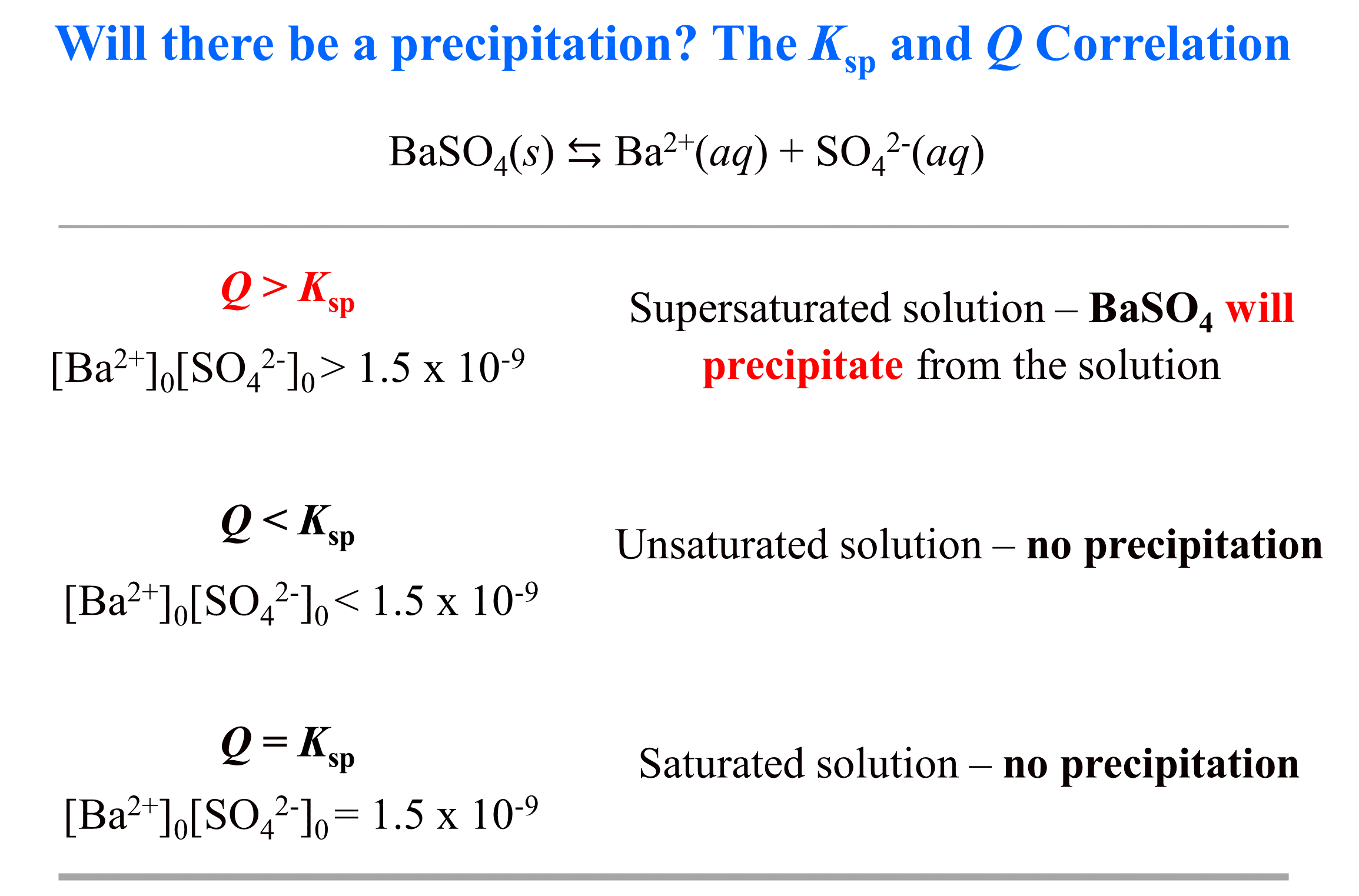
- Ksp and Molar Solubility
- The Effect of a Common Ion on Solubility
- The Effect of pH on Solubility
- Will a Precipitate Form? Ksp and Q
- Ksp and Molar Solubility Practice Problems
Chemical Thermodynamics
- Standard Entropy Change (𝚫Sorxn) of a Reaction
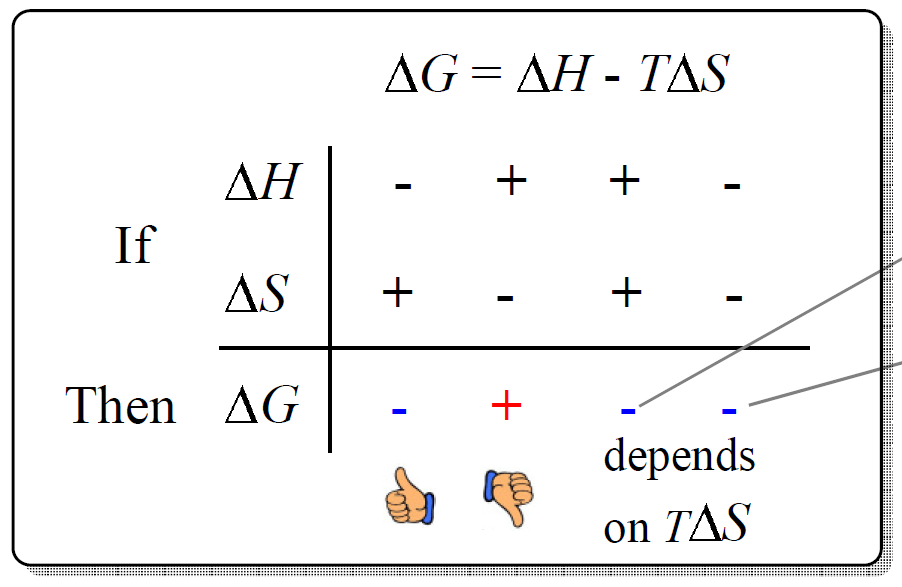
- The Gibbs Free Energy
- The Effect of 𝚫H, 𝚫S, and T on 𝚫G – Spontaneity
- Entropy and State Change
- Entropy Changes in the Surroundings
- 𝚫Gorxn from the Free Energies of Formation
- Gibbs Free Energy and Hess’s Law
- Gibbs Free Energy Under Nonstandard Conditions
- Gibbs Free Energy and Equilibrium Constant
- Entropy, Enthalpy, and Gibbs Free Energy Practice Problems
- Chemical Thermodynamics Quiz
Electrochemistry
- Balancing Redox Reactions
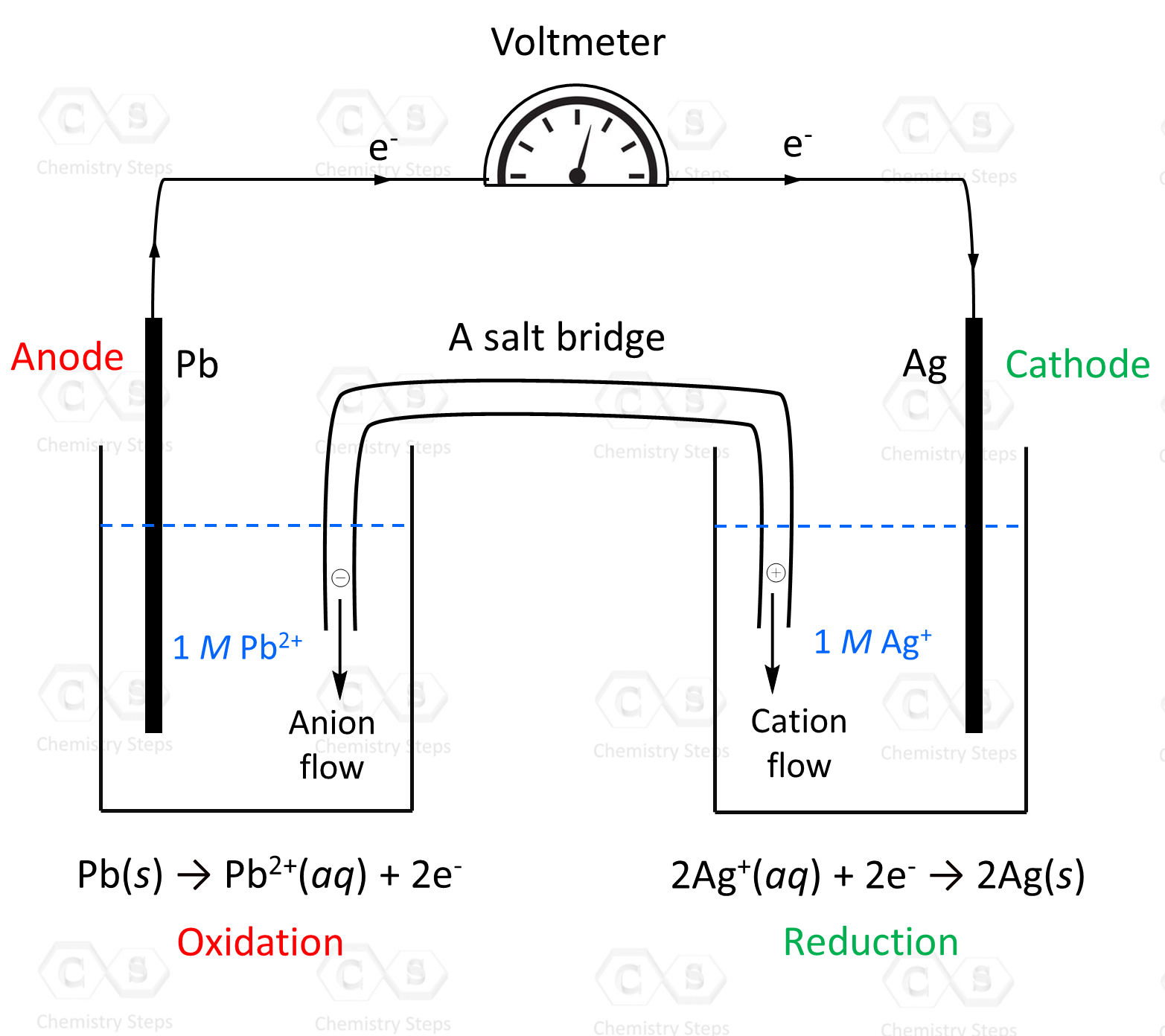
- Galvanic Cells
- How to Calculate Standard Cell Potential
- The Correlation Between Eocell, ΔG°, and K
- Nernst Equation
- Nernst Equation Practice Problems
- Concentration Cells
- Electrolytic Cells
- Electrolysis
- Electrolysis of Water
- Calculating the Mass of Metal in Electroplating
- Cell Potential Practice Problems
- Eo, ΔGo, K – Practice Problems
- Electrochemistry Practice Problems
- Electrochemistry Quiz
Nuclear Chemistry
Solutions to Over 1000 Practice Questions


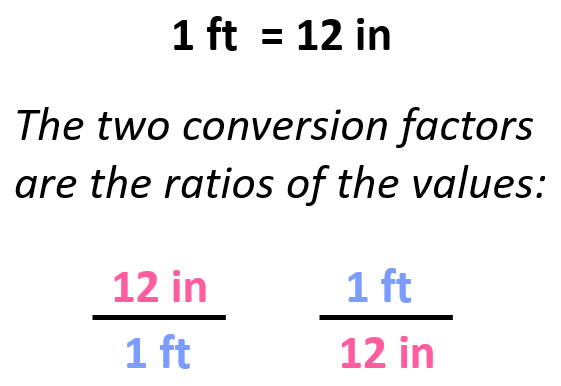
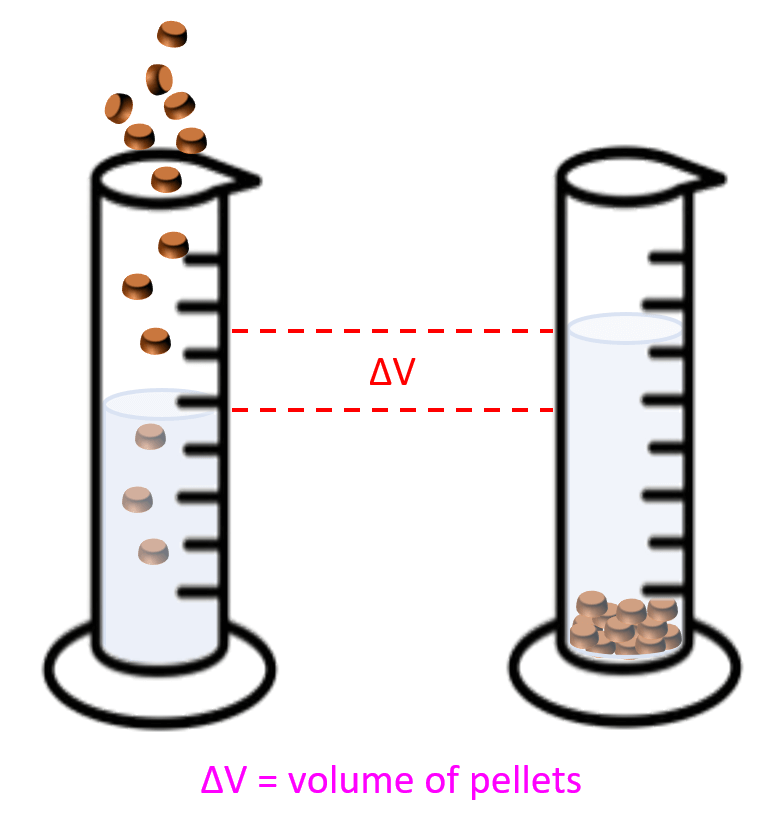 Density Practice Problems
Density Practice Problems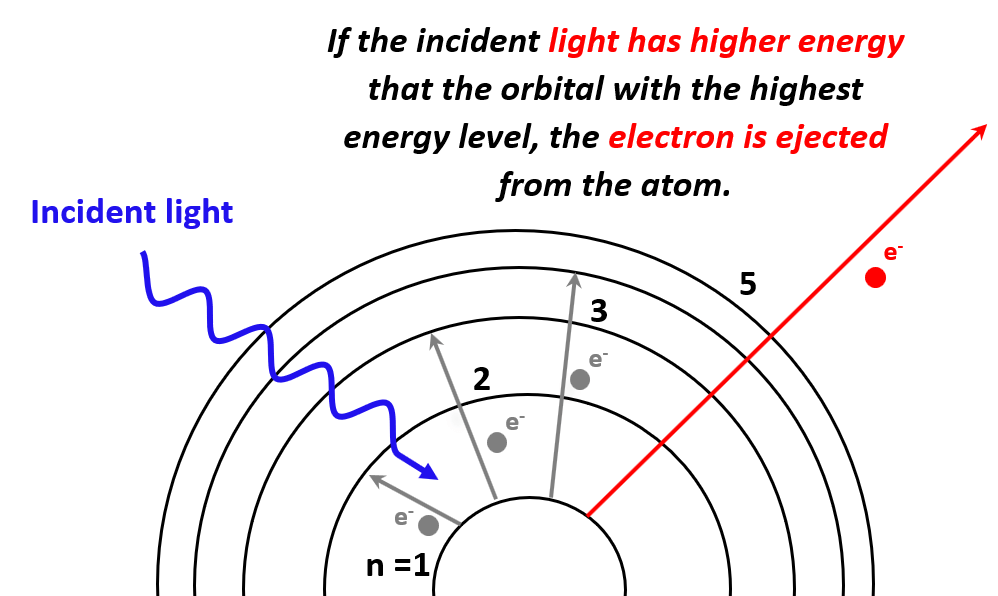

 Mass, Moles, and Number of Particles
Mass, Moles, and Number of Particles