In the previous post about solutions, we mentioned that all solutes that dissolve in water are classified as electrolytes or nonelectrolytes. Electrolytes are substances that dissolve in water to form solutions that conduct electricity.
The aqueous solutions of nonelectrolytes, on the other hand, do not conduct electricity.

And we also said that this is due to dissociation of electrolytes into ions which support the electrical circuit when dissolved in water while nonelectrolytes do not dissociate, and their molecules stay intact.
Nonelectrolytes are molecular compounds where the atoms are connected only by covalent bonding and therefore, no ions are formed when they dissolve in water. Some examples of nonelectrolytes are methanol (CH3OH), ethanol (CH3CH2OH), glucose (C6H12O6), sucrose (C12H22O11), acetone, urea ((NH2)2CO) and etc.
Again, do not confuse nonelectrolytes with insoluble compounds. Nonelectrolytes do dissolve in water, however, they do not produce ions through dissociation. Insoluble compounds do not dissolve in water.
Solubility of Inorganic Compounds
At this point we know that both electrolytes and nonelectrolytes are water-soluble. So, the question is how do we know if the compound is water-soluble or not?
To determine this, use the following rules for different combination of cations and anions that make the salt:
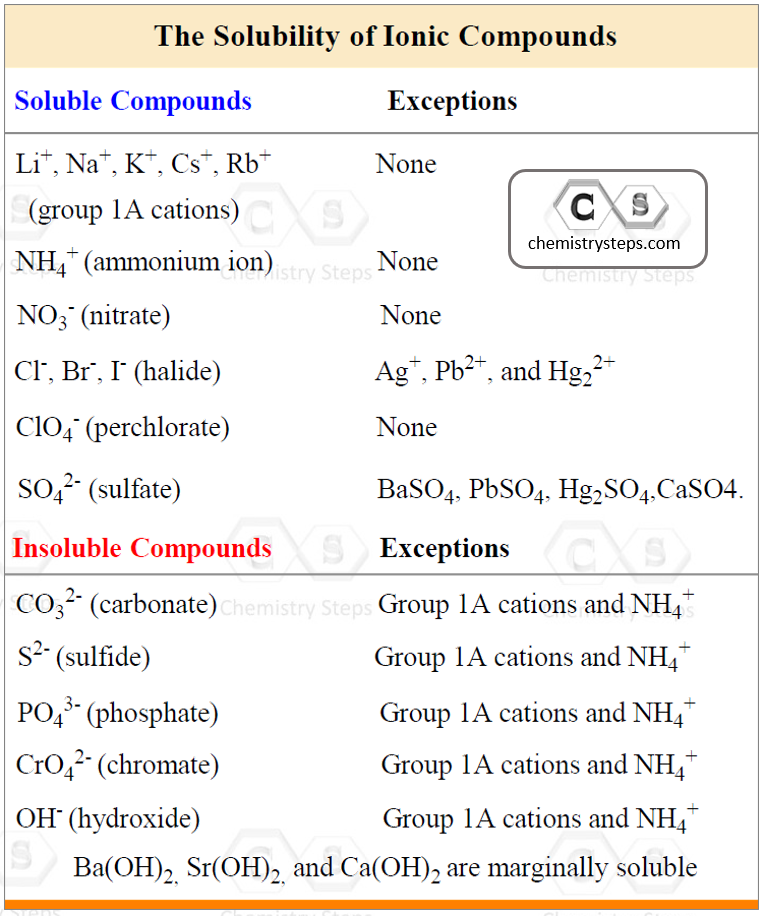
Using the information in the table, let’s determine, for example, if Ca(ClO4)2 is soluble or not. The perchlorate ion (ClO4–) is listed as soluble with any cation without exceptions. Therefore, it is a soluble salt. This, in turn, indicates that Ca(ClO4)2 is going to dissociate into ions because it is an ionic compound:
Ca(ClO4)2(aq) → Ca2+(aq) + 2ClO4–(aq)
Do not dissociate a compound that is insoluble! For example, the sulfate ion (SO42-) is listed under the soluble compounds which means most of its salts and acids are soluble. However, under the exceptions, we see that BaSO4, for example, is insoluble, and therefore, it does not dissociate in water.
We will cover the rules of writing dissociation equations in a separate post to be found here.
Strong and Weak electrolytes
Depending on the extent of the dissociation, electrolytes are classified as strong and weak. Strong electrolytes are completely ionized/dissociated in aqueous solutions, while electrolytes are largely in a molecular form and only 1-5% dissociated. For example, acetic acid (CH3CO2H), or hydrofluoric acid (HF) are weak electrolytes and only 1% -dissociated in water.
The dissociate equation of strong electrolytes in shown with a single arrow, while for weak electrolytes, a doble arrow is used indicating that the process is at equilibrium:
strong electrolyte: HCl(aq) → H+(aq) + Cl–(aq)
weak electrolyte: HF(aq) ⇆ H+(aq) + F–(aq)
weak electrolyte: CH3COOH(aq) ⇆ H+(aq) + CH3COO–(aq)
Strong acids are strong electrolytes because they produce lots of H+ ions in water and by definition the amount of protons produced by the acid is the indicator of its acid strength. Weak acids are weak electrolytes because their poor dissociation does not allow forming protons in great quantity.
In most textbooks, weak electrolytes are marked as marginally soluble in aqueous solutions.
Check Also
- Solutions
- Dissociation of Ionic Compounds
- Molecular, Ionic, and Net Ionic Equations
- Molarity
- Dilution
- Ion Concentration
- Precipitation Reactions
- Definitions of Acids and Bases
- Acid-Base Reactions
- Stoichiometry of Reactions in Aqueous Solutions
- Acid-Base Titrations
- Oxidation State
- Oxidation-Reduction (Redox) Reactions
- Reactions in Aqueous Solutions Practice Problems
