In this post, we will determine the oxidation numbers in the permanganate ion, MnO4–.
To determine the oxidation number of an atom(s) in a molecule or an ion, start with the known oxidation numbers and the rules summarized below:
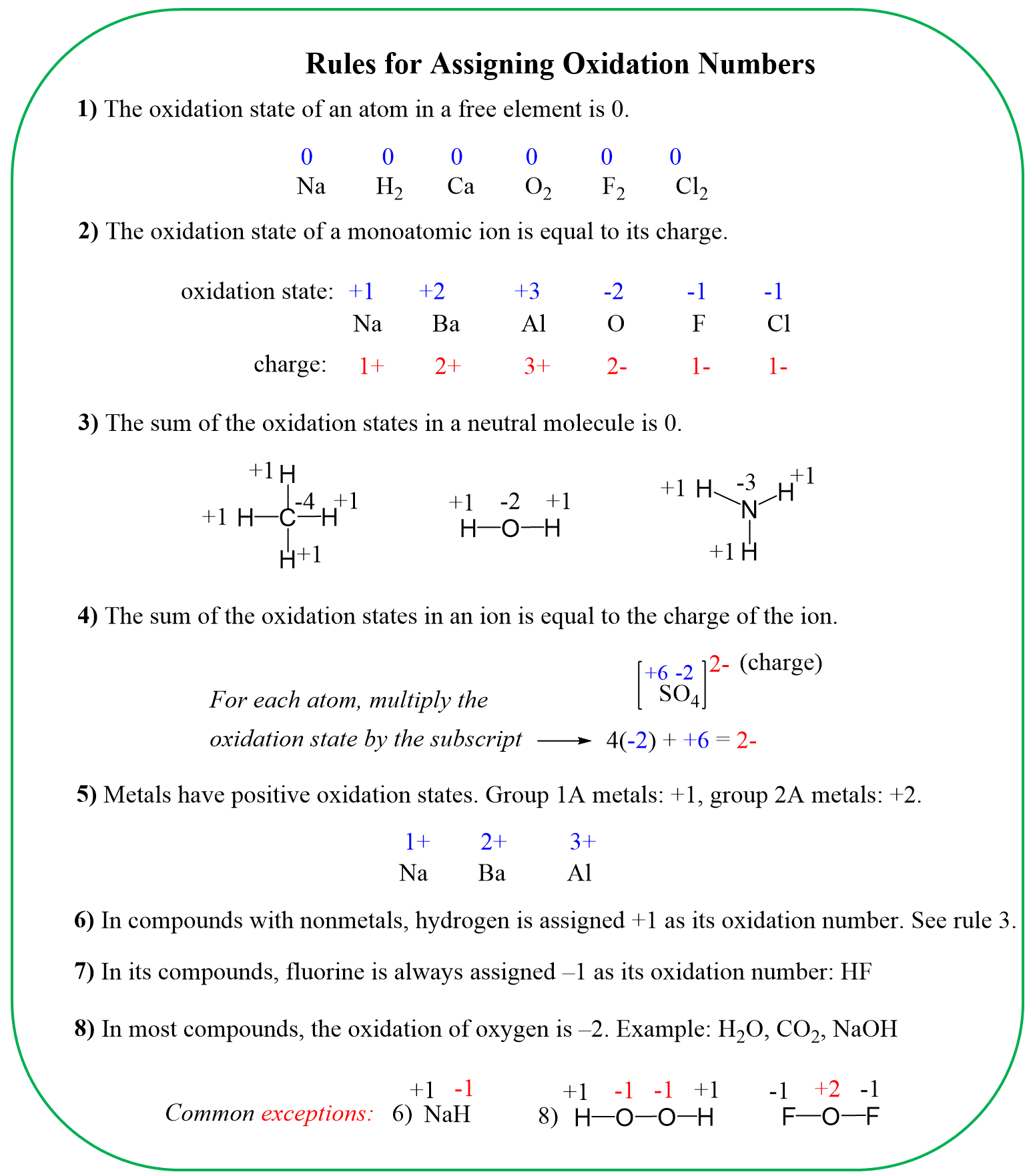
Keep in mind that the summary is zero for neutral molecules and is equal to the charge for ions.
For MnO4–the element with a standard oxidation state is oxygen which is -2, and we need to determine the oxidation state of the manganese.
Let’s confirm this by setting up an equation. Rule 8 – The oxidation of oxygen is –2:
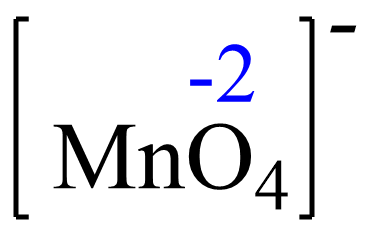
Assign x for the oxidation state of Mn and set up an equation:
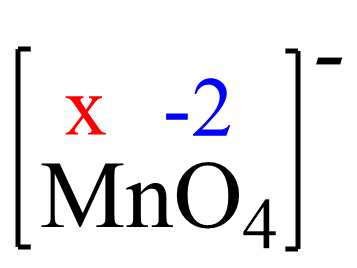
x + 4(-2) = -1, x = +7
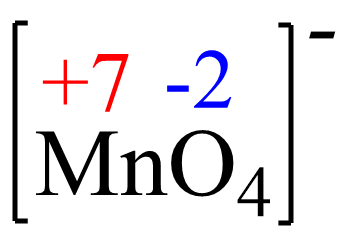
Further Reading
- Solutions
- Strong and Weak Electrolytes
- Dissociation of Ionic Compounds
- Molecular, Ionic, and Net Ionic Equations
- Molarity
- Dilution
- Ion Concentration
- Precipitation Reactions
- Definitions of Acids and Bases
- Naming Acids and Bases
- Acid-Base Reactions
- Displacement Reactions
- Predicting The Products of Chemical Reactions
- Stoichiometry of Reactions in Aqueous Solutions
- Acid-Base Titrations
- Oxidation State
- Oxidation-Reduction (Redox) Reactions
- Balancing Redox Reactions
Reactions in Aqueous Solutions – Practice Problems
More examples of the oxidation state in this multiple-choice quiz:

