In this post, we will determine the oxidation numbers in the phosphate ion – PO43-.
To determine the oxidation number of an atom(s) in a molecule or an ion, start with the known oxidation numbers and the rules summarized below:
Keep in mind that the summary is zero for neutral molecules and is equal to the charge for ions.
For PO43-, the element with a standard oxidation state is oxygen which is -2, and we need to determine the oxidation state of the sulfur.
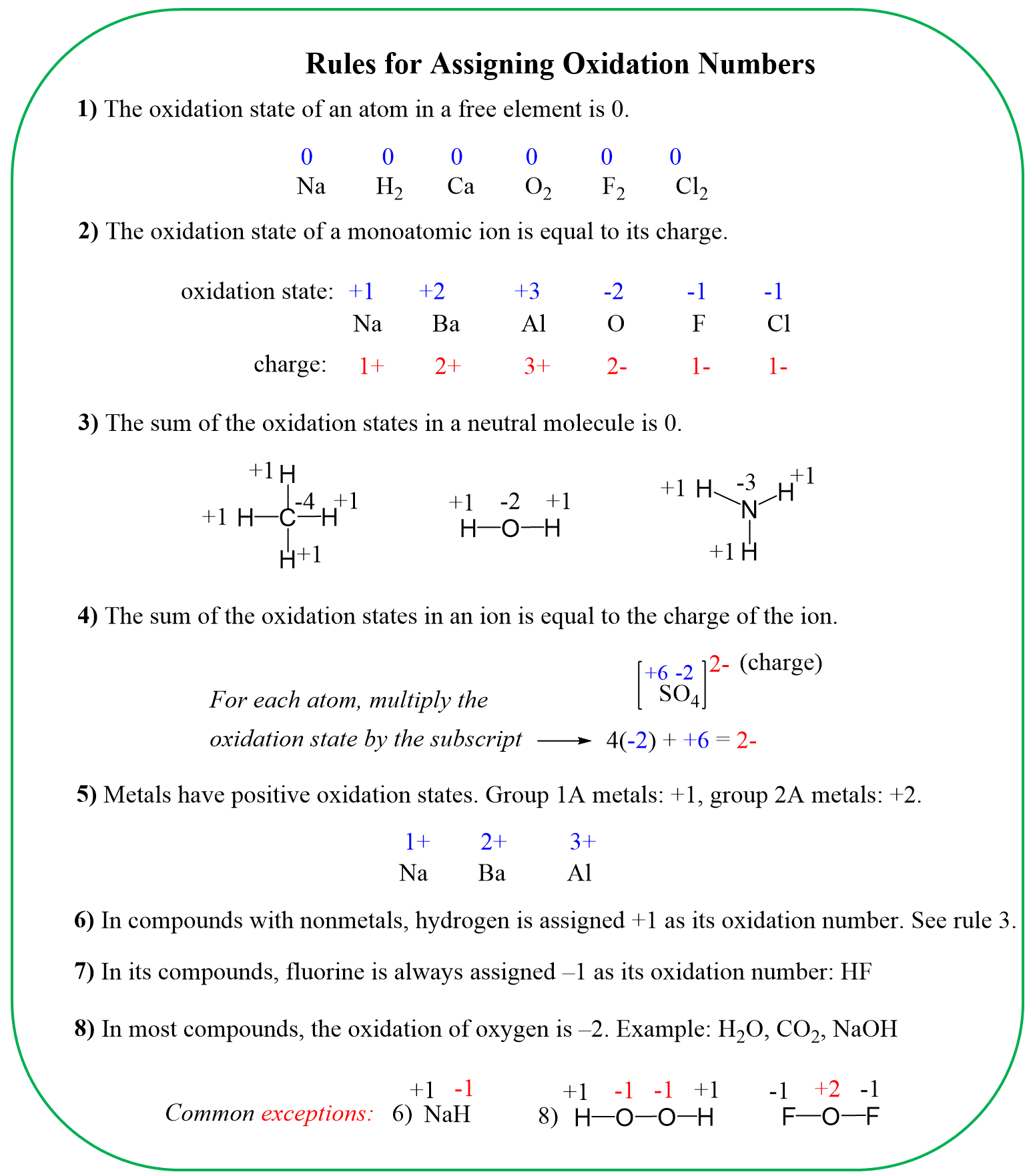
Rule 8 – The oxidation of oxygen is -2:
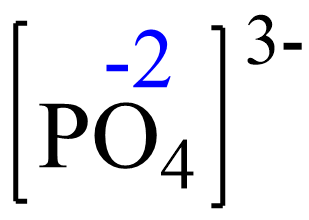
Assign x for the oxidation state of P and set up an equation:
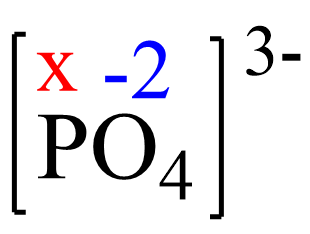
x + 4(-2) = -3, x = +5
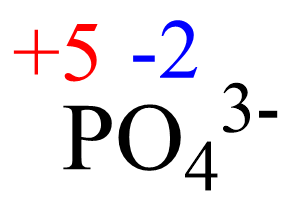
The oxidation number of the element in the given ion is the same for any salt containing that ion. For example, we can confirm that the oxidation number phosphorus in Ca3(PO4)2 is +5 because it is +5 in the phosphate ion.
Based on rule 2, the oxidation number of calcium in Ca2+ is +2, and that of oxygen is -2 according to rule 8. So, we can set up the following equation:
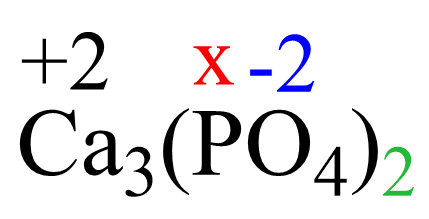
3(+2) + 2(x) + 2 x 4 x (-2) = 0
x = +5
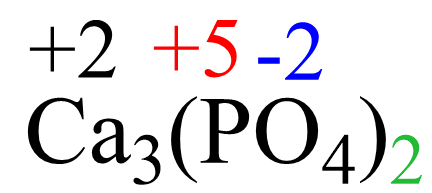
To confirm the answer:
3(+2) + 2(+5) + 2 x 4 x (-2) = 0
Further Reading
- Solutions
- Strong and Weak Electrolytes
- Dissociation of Ionic Compounds
- Molecular, Ionic, and Net Ionic Equations
- Molarity
- Dilution
- Ion Concentration
- Precipitation Reactions
- Definitions of Acids and Bases
- Naming Acids and Bases
- Acid-Base Reactions
- Displacement Reactions
- Predicting The Products of Chemical Reactions
- Stoichiometry of Reactions in Aqueous Solutions
- Acid-Base Titrations
- Oxidation State
- Oxidation-Reduction (Redox) Reactions
- Balancing Redox Reactions
Reactions in Aqueous Solutions – Practice Problems
More examples of the oxidation state in this multiple-choice quiz:

