In the previous post, we talked about atomic radii, and discussed the following periodic trends:
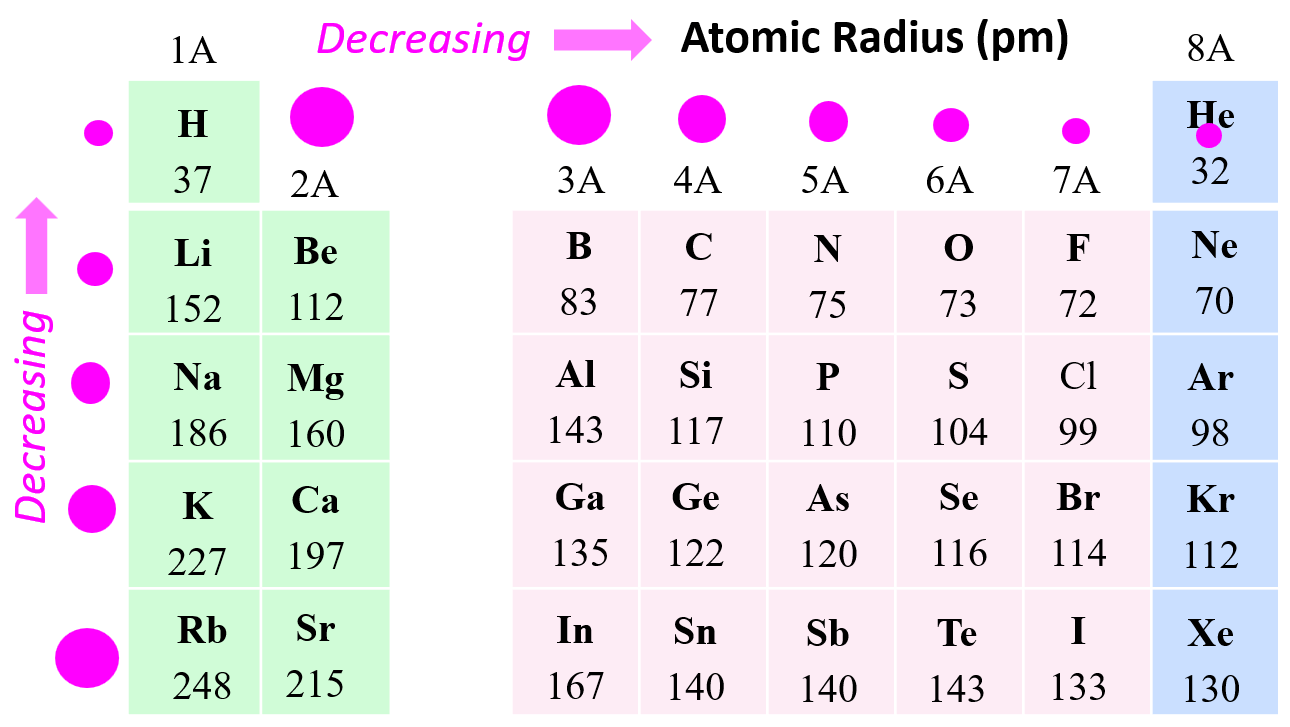
- Atomic radius decreases across the periodic table to the right.
- Atomic radius increases down a group in the periodic table.
Radii of Cations
Cations are formed when an atom loses an electron(s) which results in decreasing the radius. The radius of a cation is always smaller than the radius of the parent atom. For example, Na+ < Na, Ca2+ < Ca, etc.
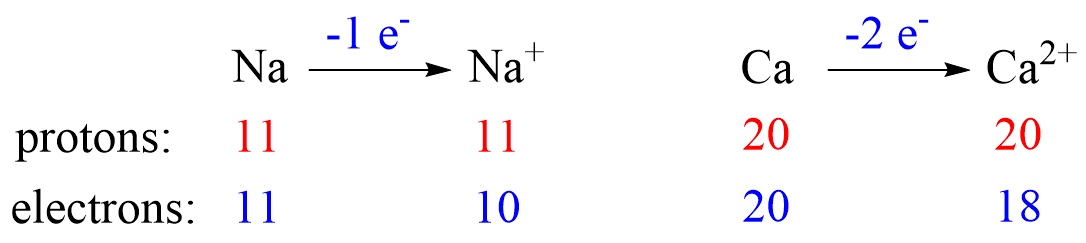
To explain this pattern, let’s compare the electron configuration of sodium and Na+ ion.
The electron configuration of sodium is 1s22s22p63s1, and the electron is removed from the energy level with the greatest n value – 3s. Therefore, the electron configuration of the Na+ ion will be 1s22s22p6. The 3s1 is the valence electron of sodium, which compared to the core electrons is a lot farther from the nucleus and removing it decreases the radius significantly (from 186 pm to 95 pm):
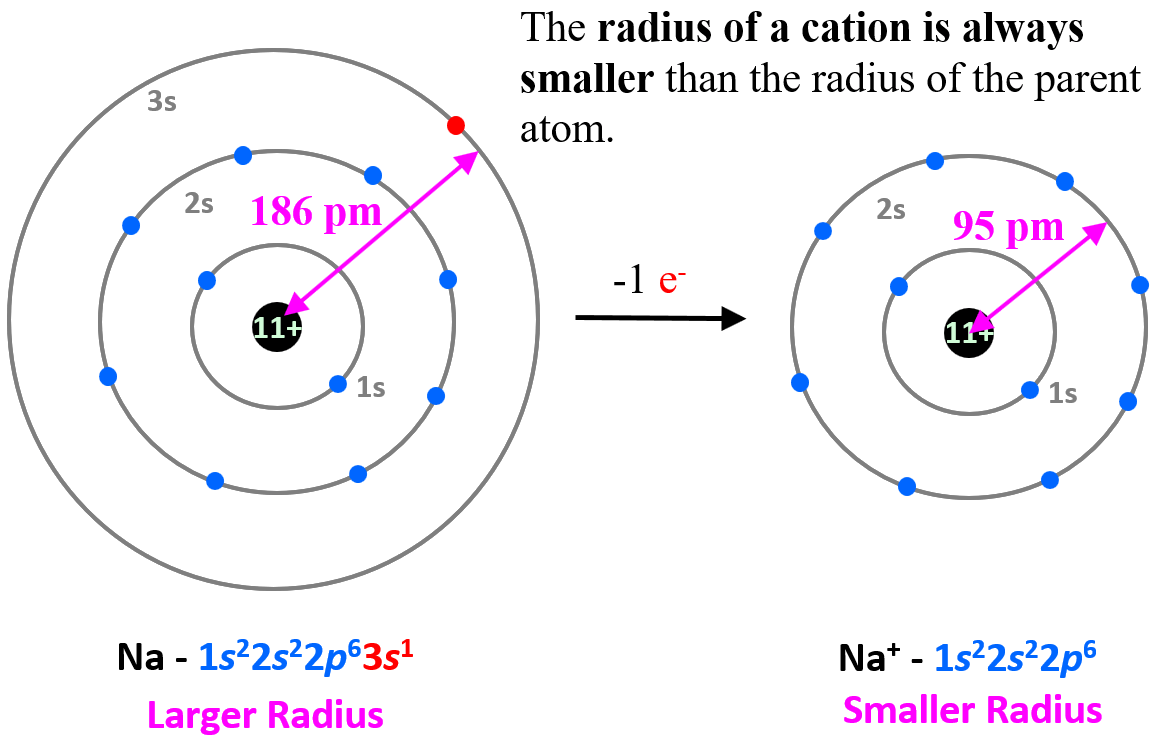
This pattern is true for all the cations and their atoms.
Radii of Anions
The opposite trend is observed for anions which are always larger than their parent atoms. For example, Cl– > Cl, S2- > S. Although the newly added electron(s) does not go into a higher energy level, it increases the repulsive interactions among the electrons which increases the radius.
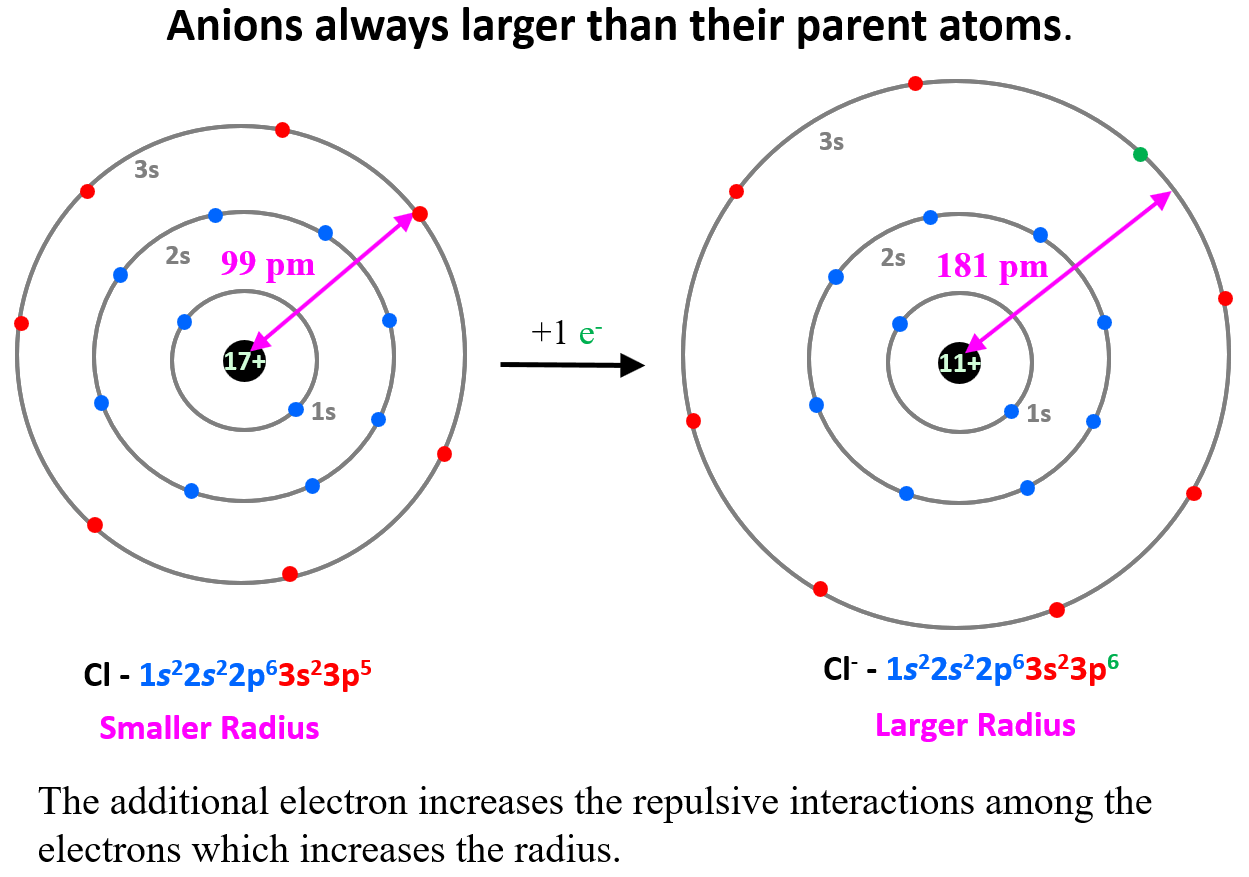
This increase would have been somewhat balanced if the atomic number, meaning the nuclear charge, increased as well, but we are comparing the radii of the same element.
Atomic Radii of Isoelectronic Species
Let’s compare the radii of species that have the same number of electrons but a different number of protons (isoelectronic species). For example, K+, Ca2+, Cl-, and S2- have 18 electrons, however, their radii are not the same because they are different elements and therefore, have a different nuclear charge.
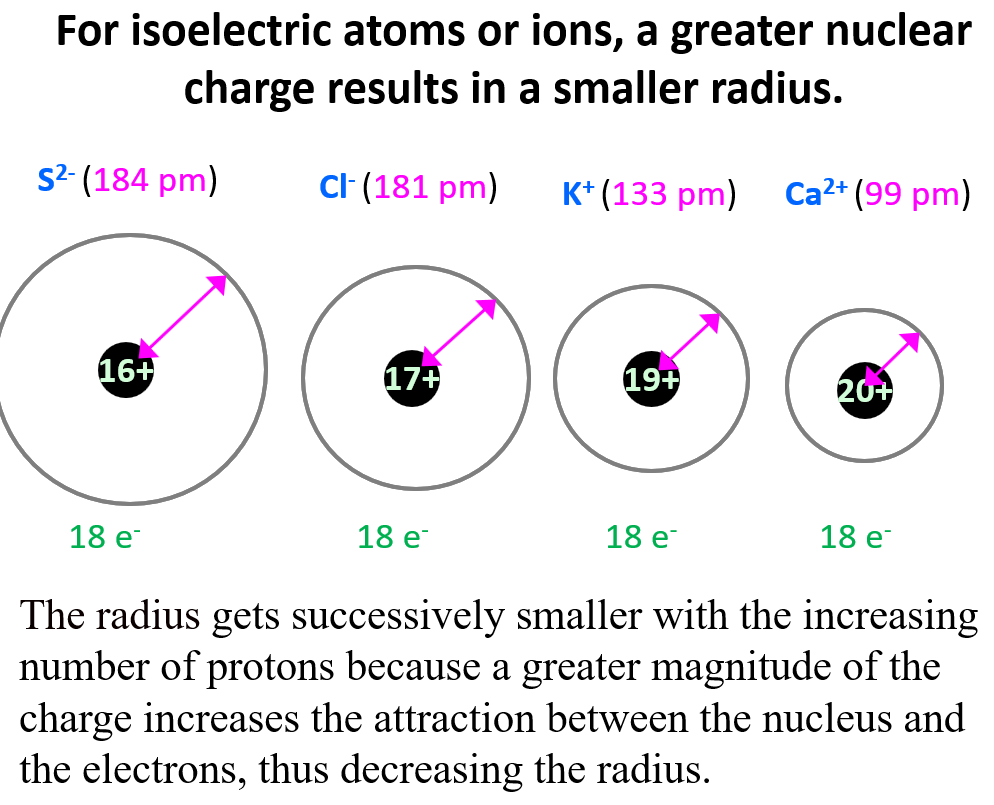
Notice that the radius gets successively smaller with the increasing number of protons because, as we discussed in the article about effective nuclear charge, a greater magnitude of the charge increases the attraction between the nucleus and the electrons, thus decreasing the radius.
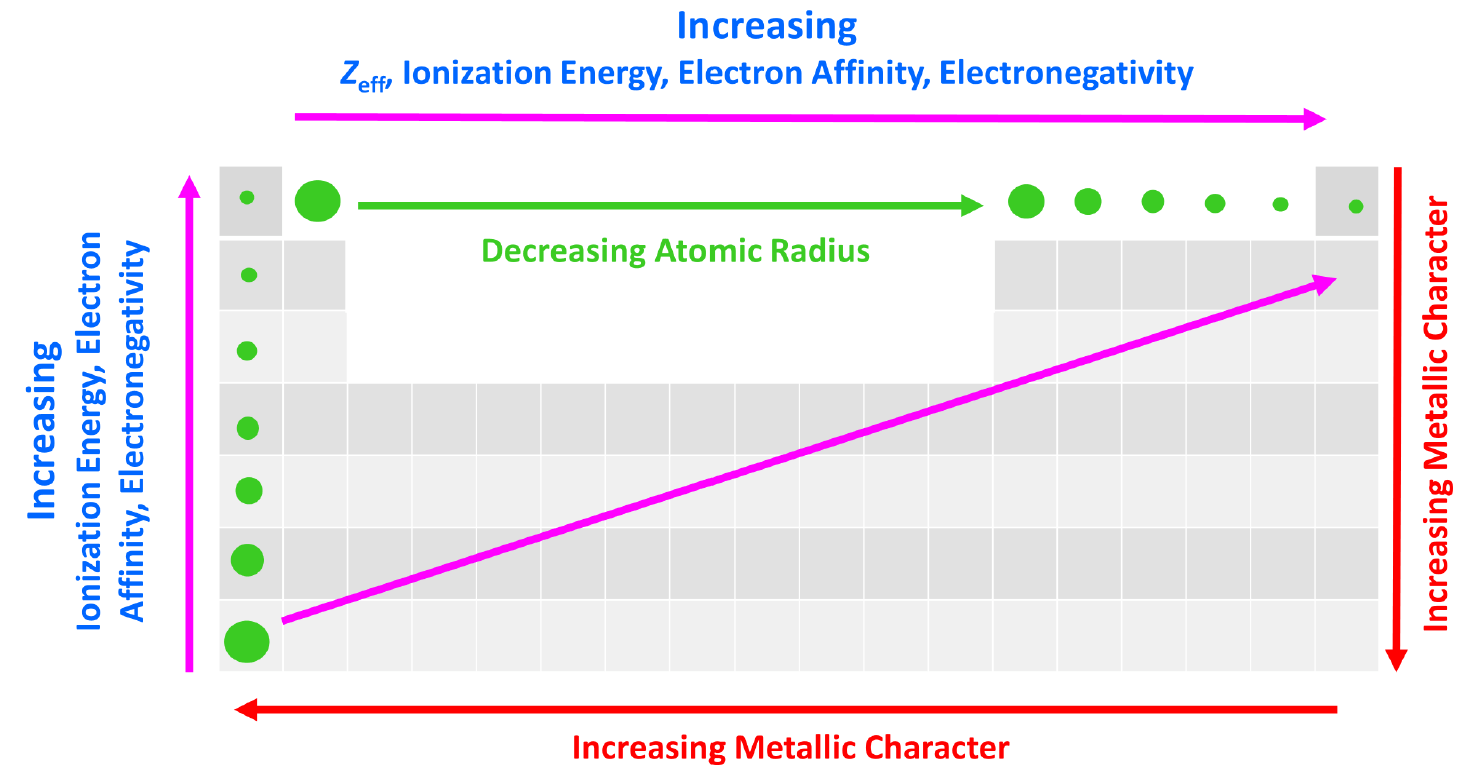
The effective nuclear charge is responsible for many properties of the elements such as electronegativity, ionization energy, atomic radius, etc. The stronger the effective nuclear charge, the smaller the atom and thus the stronger the attractive forces between the nucleus and the valence electrons which means the higher the ionization energies, electron affinity, and electronegativity.
We can put together a general trend for the atomic radius, effective nuclear charge, ionization energy, electron affinity, and electronegativity.
You can look at these as how much an atom ‘likes’ electrons. The more we move to the right in the periodic table, the more the atoms like electrons, because of a greater nuclear charge which increases the ionization energy, electron affinity, and electronegativity. This results in smaller atoms because the nucleus holds the electrons closer and tighter.
This, in turn, means that the metallic character of the elements decreases as metals ‘don’t like’ electrons and tend to become cations.
Check this 95-question, Multiple-Choice Quiz on the Electronic Structure of Atoms including questions on properties of light such as wavelength, frequency, energy, quantum numbers, atomic orbitals, electron configurations, and more.
Check Also
- Atomic Orbitals
- Electron Configurations
- Electron Configurations of Ions
- Orbital Diagrams
- Aufbau’s Principle, Hund’s Rule, and Pauli’s Exclusion Principle
- Hund’s Rule
- Pauli Exclusion Principle
- Quantum Numbers (n, l, ml, ms)
- Bohr Model of the Hydrogen Atom
- Rydberg Formula
- The Photoelectric Effect
- Calculating The Energy of a Photon
- Effective Nuclear Charge
- Atomic Radius
- Ionization Energy
- Electron Affinity
- Energy, Wavelength, and Frequency Practice Problems

