To determine the oxidation number of an atom(s) in a molecule or an ion, start with the known oxidation numbers and the rules summarized below:
Keep in mind that the summary is zero for neutral molecules and is equal to the charge for ions.
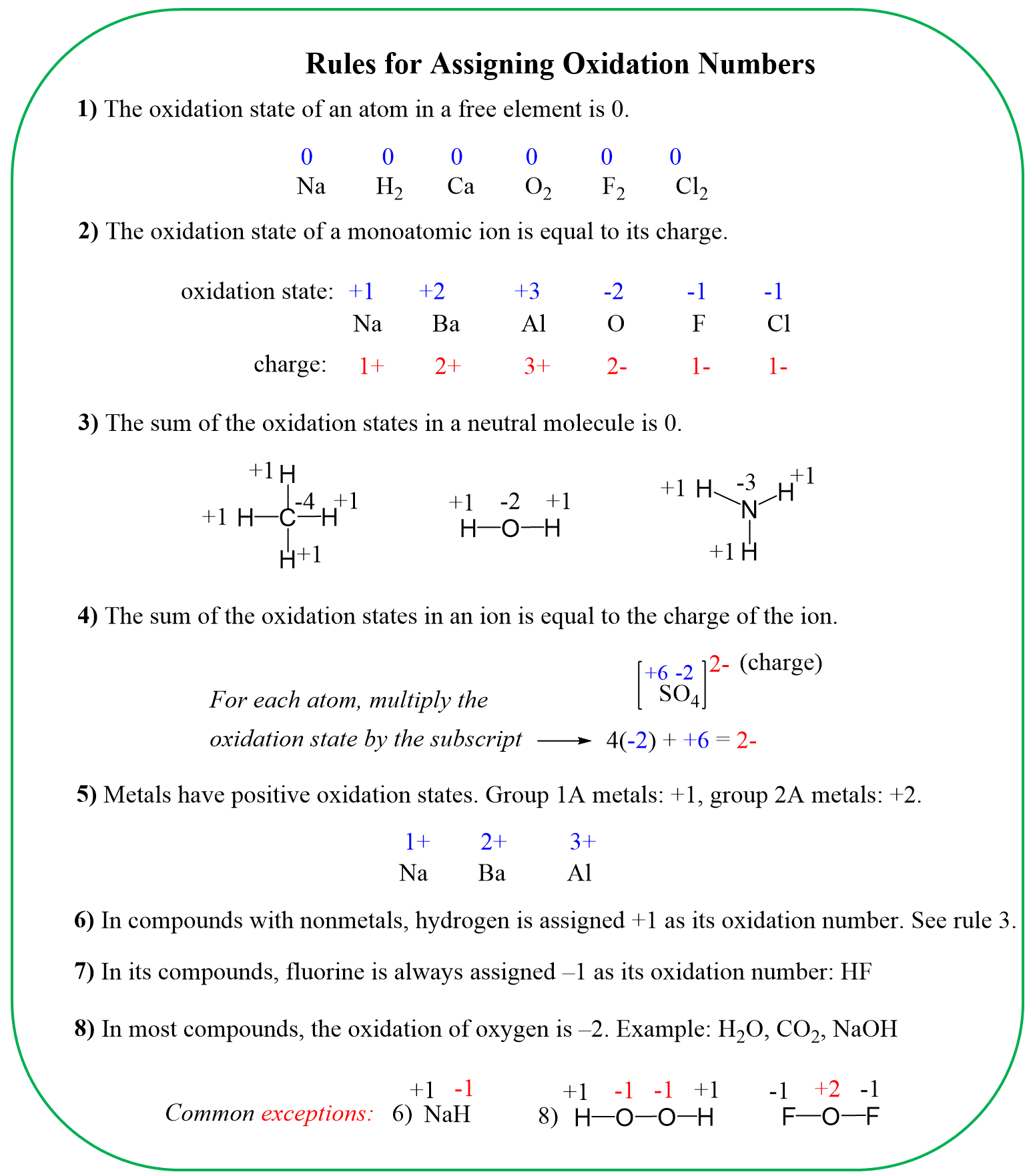
For the dichromate ion, Cr2O72- the element with a standard oxidation state is oxygen which is -2, and we need to determine the oxidation state of the chromium.
Rule 8 – The oxidation of oxygen is –2:
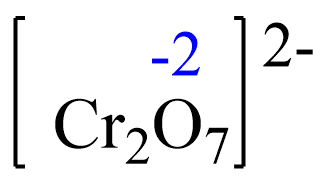
Assign x for the oxidation state of Cr and set up an equation. Remember, for ions, the sum of the oxidation numbers multiplied by their subscripts must be equal to the charge of the ion:
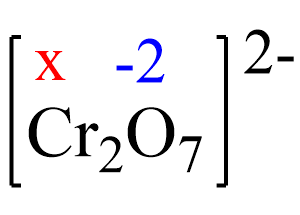
2x + 7(-2) = -2, x = +6
Further Reading
- Solutions
- Strong and Weak Electrolytes
- Dissociation of Ionic Compounds
- Molecular, Ionic, and Net Ionic Equations
- Molarity
- Dilution
- Ion Concentration
- Precipitation Reactions
- Definitions of Acids and Bases
- Naming Acids and Bases
- Acid-Base Reactions
- Displacement Reactions
- Predicting The Products of Chemical Reactions
- Stoichiometry of Reactions in Aqueous Solutions
- Acid-Base Titrations
- Oxidation State
- Oxidation-Reduction (Redox) Reactions
More examples of the oxidation state in this multiple-choice quiz:

