In the previous post, we talked about conversion factors and their use in dimensional analysis. Remember, in short, the conversion factors are the ratios of the units for the given quantity.
For example, we know that 1 ft is 12 in, and therefore, we can derive two conversion factors by simply getting the ratio of these units:
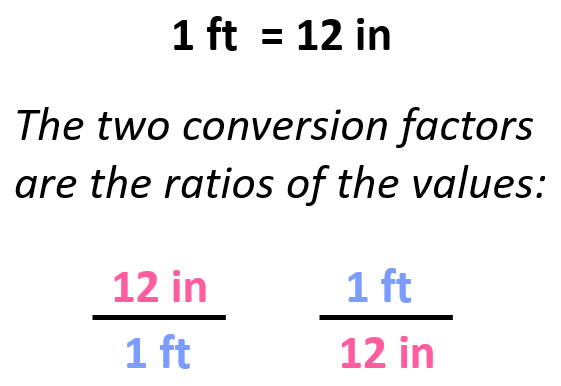
Once we have the conversion factor, we can convert a quantity given in one unit into an equivalent quantity with a different unit by multiplying it with the correct factor, which is the one that allows canceling the initial units.
Let’s say we need to calculate how many inches 4.58 ft is.
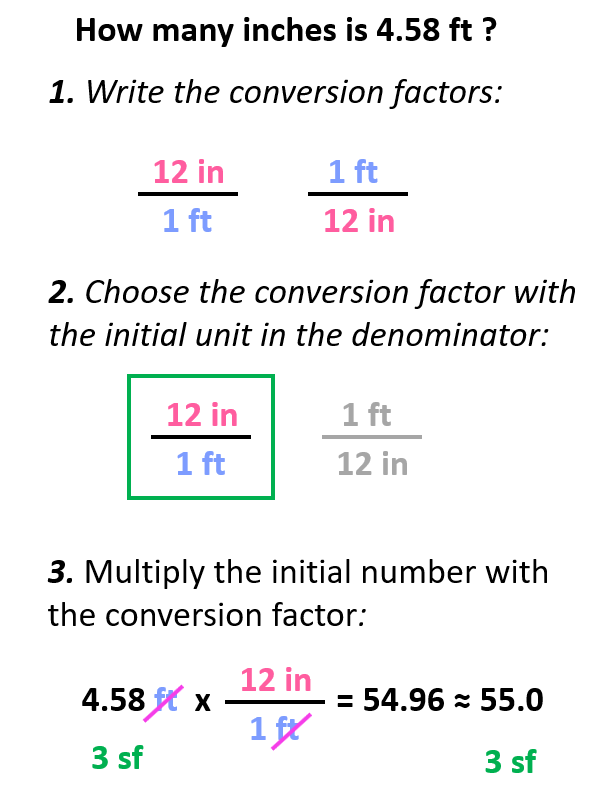
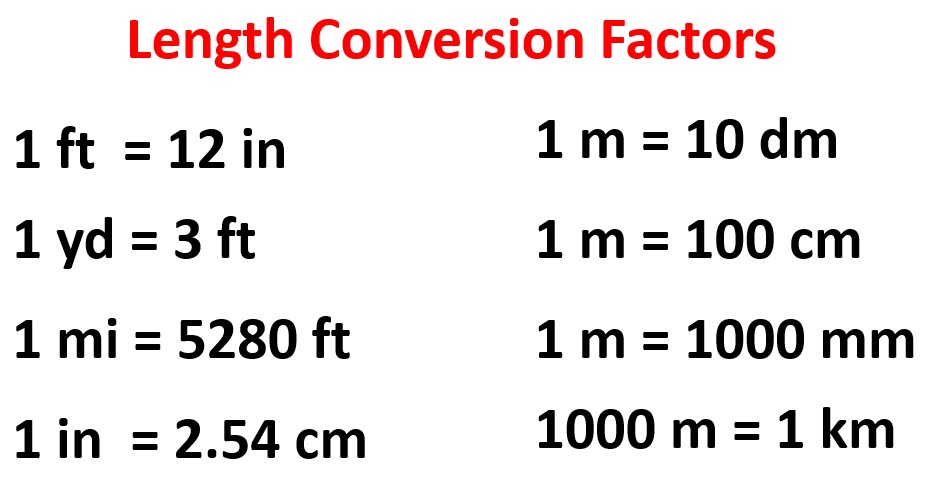
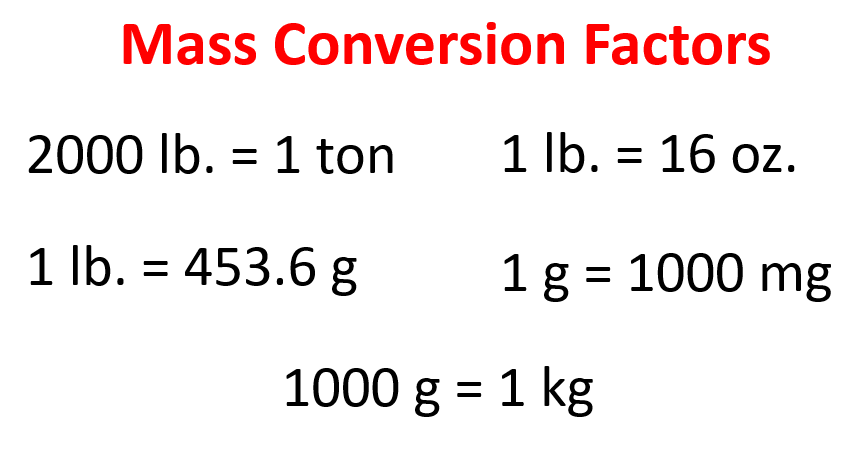
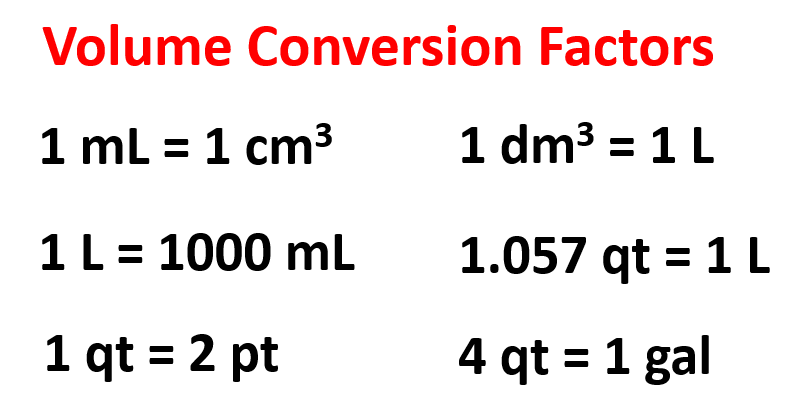
Below are some of the common conversion factors for length, mass, and volume that you can use to work on the practice problems.
Check Also
- Significant Figures
- Significant Figures in Addition, Subtraction Multiplication, and Division
- Significant Figures Practice Problems
- Converting Units With Conversion Factors Dimensional Analysis
- Density Practice Problems
Practice
Perform each of the following conversions.
a) 5.98 cm to millimeters
b) 65 cm to meters
c) 4.87 mm to inches (1 in = 2.54 cm, 1 cm = 10 mm)
d) 2894 ft to kilometers (1 mi = 1.609 km)
e) 6854 m to miles (1 mi = 1.609 km)
f) 548 lb to kilograms
g) 451 oz to kilograms
h) 1564 mL to gallons (1 gal = 3.785 L)
i) 659 mL to quarts
j) 72 gal to milliliters
k) 482 lb to grams
l) 54 quarts to milliliters
How many weeks are in 2.5 centuries? (1 yr. = 52 weeks)
Units Raised to a Power
Perform each of the following conversions.
a) 45 cm2 to in2
b) 59 mm2 to ft2
c) 995 ft2 to m2
d) 2.54 dm3 to in3 (1 dm = 10 cm)
e) 4.22 gallons to cm3 (1 L = 1000 cm3)
f) 5.64 L to ft3
Would 550 m2 of fabric be enough to upholster 200 chairs if each requires 35.4 ft2 fabric?
Convert 16.4 cubic decimeters (16.4 dm3) to gallons, using the following conversion factors:
2.54 cm = 1 in, 231 in3 = 1 gallon, 1 dm = 10 cm
Converting Two Units
8) Perform each of the following conversions.
a) 195 cm/s to mi/h
b) 55 km/h to yd/min (1 m = 1.094 yd)
c) 45 mi/h to m/s
The world record for 100-m dash is 8.58 s. Calculate the average speed of the athlete in mi/h.
Assuming constant speed, how long will it take for the athlete to run 500. m if she runs the 100-yard dash in 12.0 s?
Ho many liters of soda will be needed for 50 guests if each of them drinks 10. fl. oz. of soda?
What is the speed of a car in cm/s if it goes 25 mi/h?
Would a car traveling at a constant speed of 62 km/h violate a 40 mi/h speed limit?
A kilogram of mandarin costs 1.45 euros. Given the exchange rate of 1 euro = 1.18 dollars, how many lb of mandaring can you by with 5.00 dollars?
Density
A 20.0-mL sample of a liquid has a mass of 17.8 g. What is the liquid’s density in grams per milliliter?
A sample containing 31.25 g of metal pellets is poured into a graduated cylinder initially containing 11.9 mL of water, causing the water level in the cylinder to rise to 18.7 mL. Calculate the density of the metal.
What is the mass of a 2.85 L sample of a liquid that has a density of 0.954 g/mL?
What is the volume of 100. g bromine in milliliters if it has a density of 3.10 g/cm3?
Complete the missing data for the density, mass, and volume in the following table:
| Mass | Volume | Density |
| 2.35 g | 0.035 L | X g/mL |
| X lb | 356 mL | 1.56 g/cm3 |
| 14.6 kg | X gal | 4.81 g/mL |
How many kilograms of honey with a density of 1.40 kg/L are there in a gallon container?
The density of iron is 8.96 g/cm3. What is its density in pounds per cubic inch (lb/in3)?
The density of iron is 7.86 g/cm3. What is the volume of 5.24 lb of iron expressed in cubic inches?
A plastic cylinder has a length of 7.25 in, a radius of 1.26 in, and a mass of 841 g. What is the density of the plastic in g/cm3?
What is the radius of a steel sphere that has a mass of 65.0 g and a density of 7.86 g/cm3?

