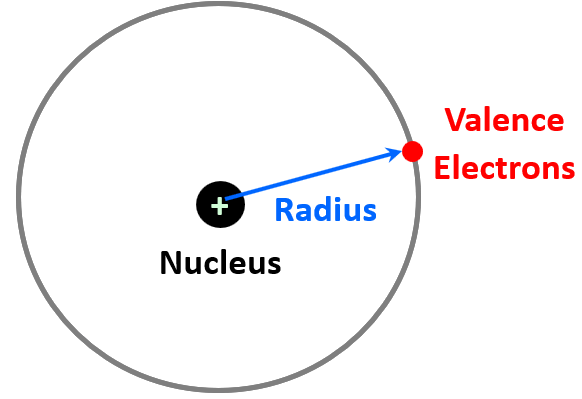
In a general definition, radius is a straight line from the center to the circumference of a circle or sphere. So, how do we define the atomic radius? The center of the atom is the nucleus, and we consider the circumference as the region where the outermost energy level electron (valence electron) spends most of its time. So, we can think of the atomic radius as the distance from the nucleus to the electron in the highest energy level.
Van Der Waals Radius
The next question is how do we measure the distance between the nucleus and this electron? For some monoatomic atoms, it is not as difficult given the modern instrumentation and calculation methods. These are mainly the noble gases and those that do not exist allotrope such as P4, S8, etc. So, if the atoms are touching but not bonding, we can determine the distance between them, and the radius would be half of that distance:
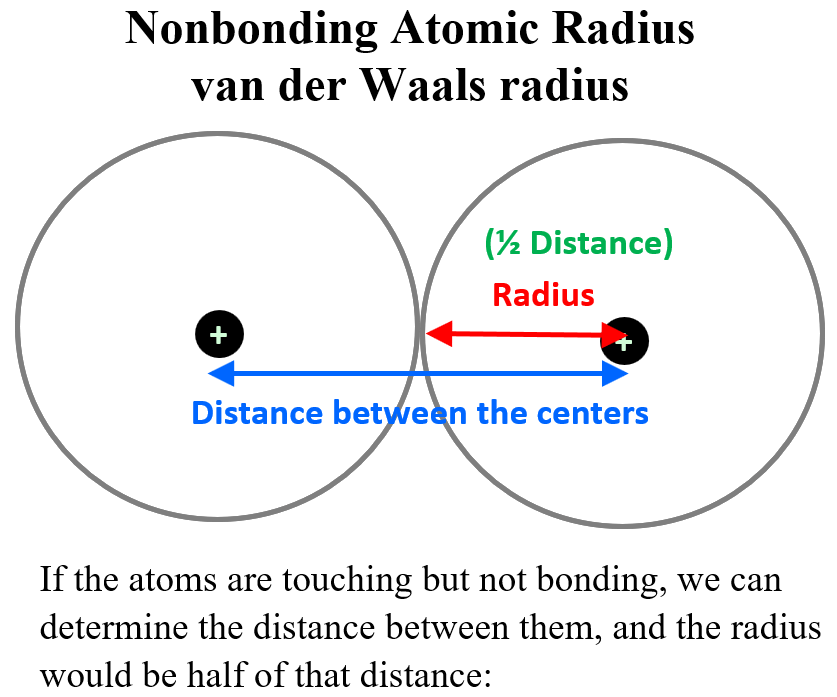
This is called the is called the nonbonding atomic radius or the van der Waals radius which represents the radius of an atom when it is not bonded to another atom.
Covalent Radius
Now, many atoms do not exist in monoatomic and isolated forms, and for these, the radius is measured by half of the distance between the nuclei of two identical atoms in a diatomic molecule.
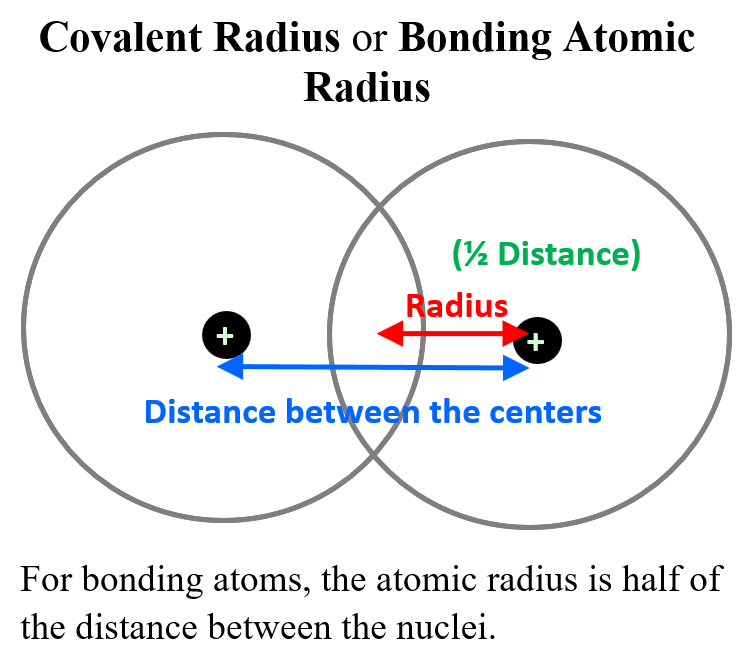
This is called the covalent radius or bonding atomic radius. For example, the Cl-Cl bond is measured to be 198 pm and therefore, the atomic radius of Cl is 99 pm (0.99 A). The covalent radius of Br is determined 114 pm since the distance between Br atoms in Br2 is 228 pm.
For metals, the atomic radius is defined as half the distance between two neighboring atoms in a crystal of the metal:
Bond Length from Atomic Radii
The bond length of a diatomic molecule consisting of two different nonmetals can be calculated by the sum of their atomic radii. For example, we can estimate the bond length for ICl by adding the atomic radius of iodine (133 pm) and chlorine’s atomic radius (99 pm) which comes to 232 pm. It is slightly shorter than the experimentally measured 232.07 pm I-Cl bond length.
Atomic Radius Periodic Trends
Atomic radius increases down the groups in the periodic table (Li < Na < K < Rb < Cs, for instance), and it decreases as we move to the right across a period in the periodic table (Li > Be > B > C > N > O > F).
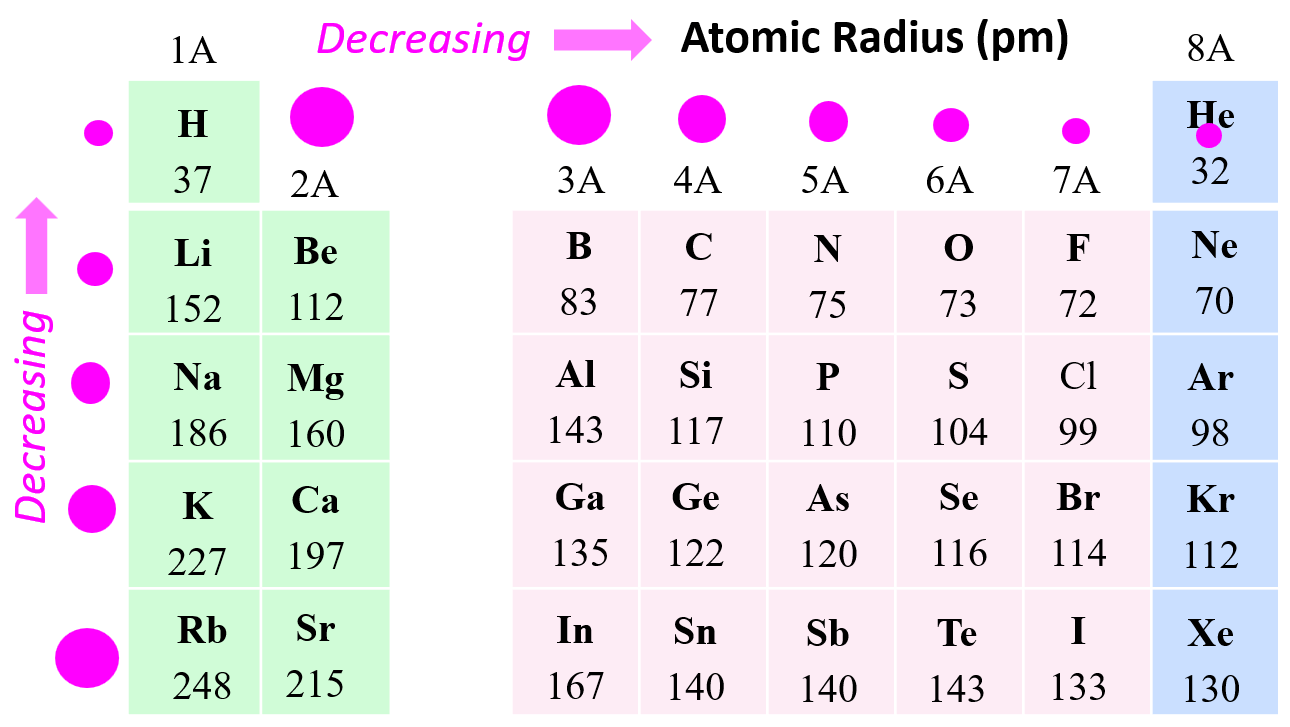
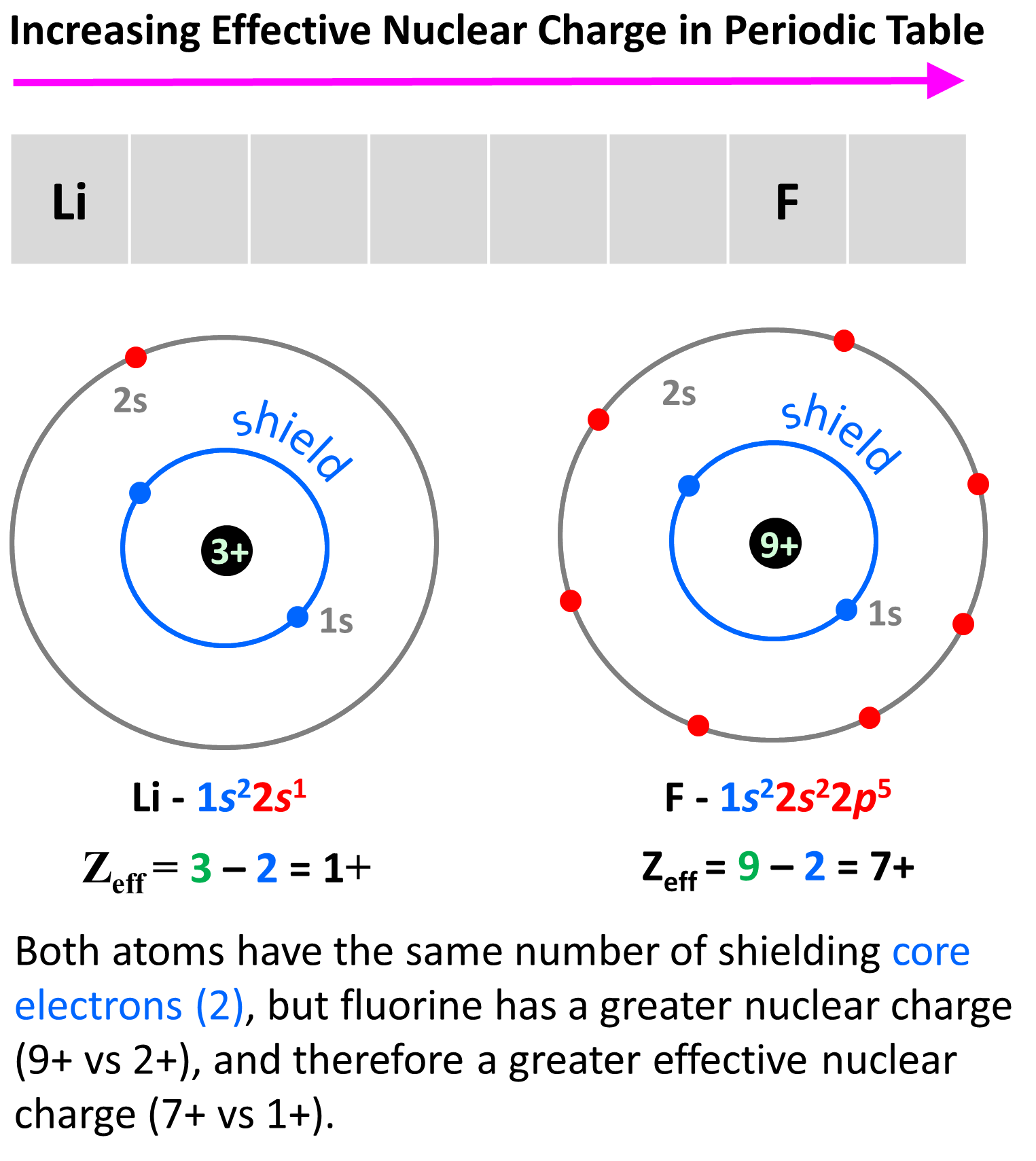
Why is the atomic radius decreasing across the groups of the periodic table? This is in correlation with the effective nuclear charge. Remember, as we move to the right, the nuclear charge increases while the number of core electrons stays constant, and as a result, the valence electrons experience a stronger attraction force with the nucleus. Yes, the number of valence electrons increases as well, but these have a significantly lower shielding effect, and each of them experiences a greater effective nuclear charge:
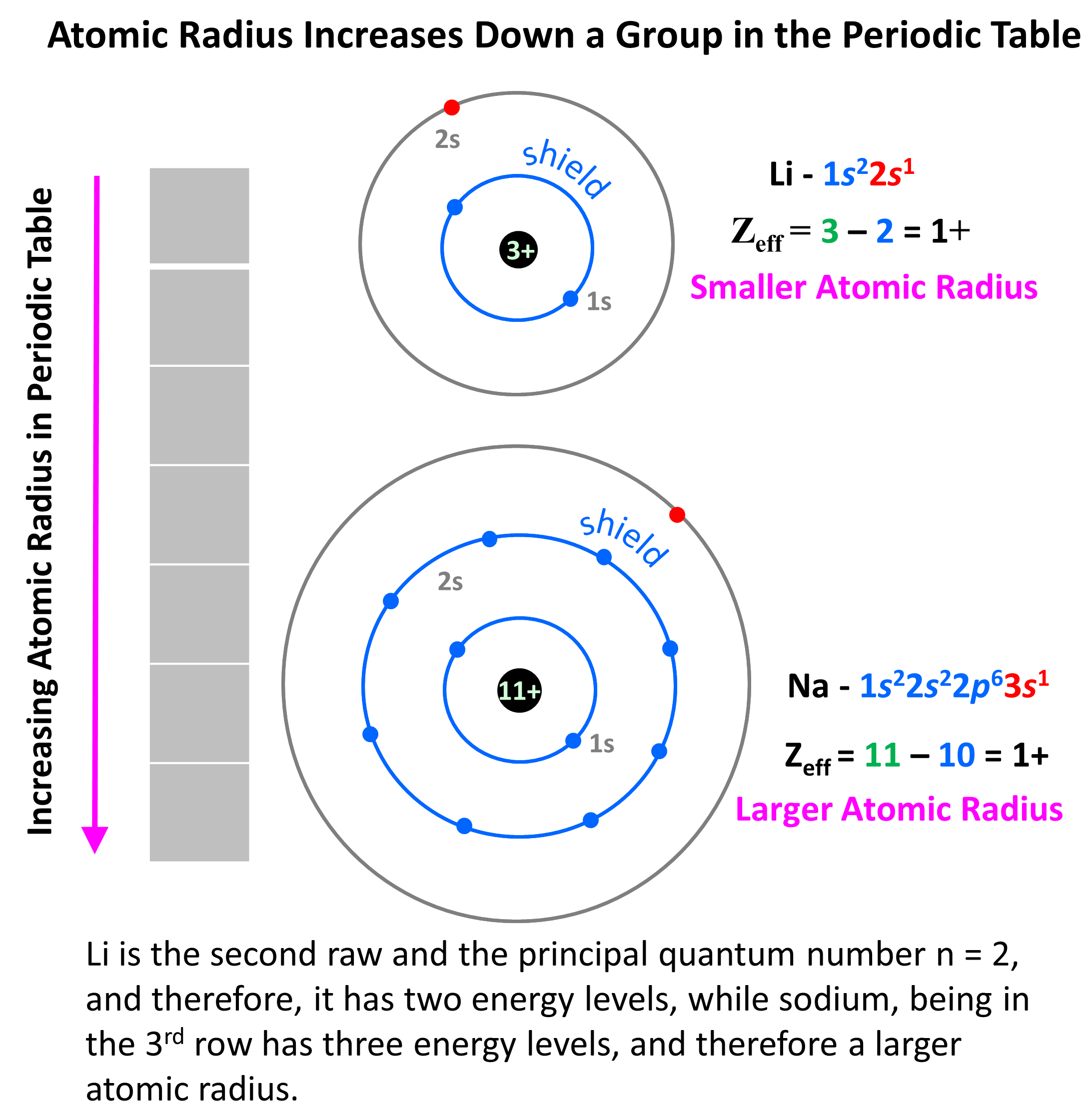
For the groups, the reason for increasing atomic radii is the increasing size of the atomic orbitals and the additional energy levels that the atom has. For example, Li is the second raw and the principal quantum number n = 2, and therefore, it has two energy levels, while sodium, being in the 3rd row has three energy levels:
The effective nuclear charge is responsible for many properties of the elements such as electronegativity, ionization energy, atomic radius, etc. The stronger the effective nuclear charge, the smaller the atom and thus the stronger the attractive forces between the nucleus and the valence electrons which means the higher the ionization energies, electron affinity, and electronegativity.
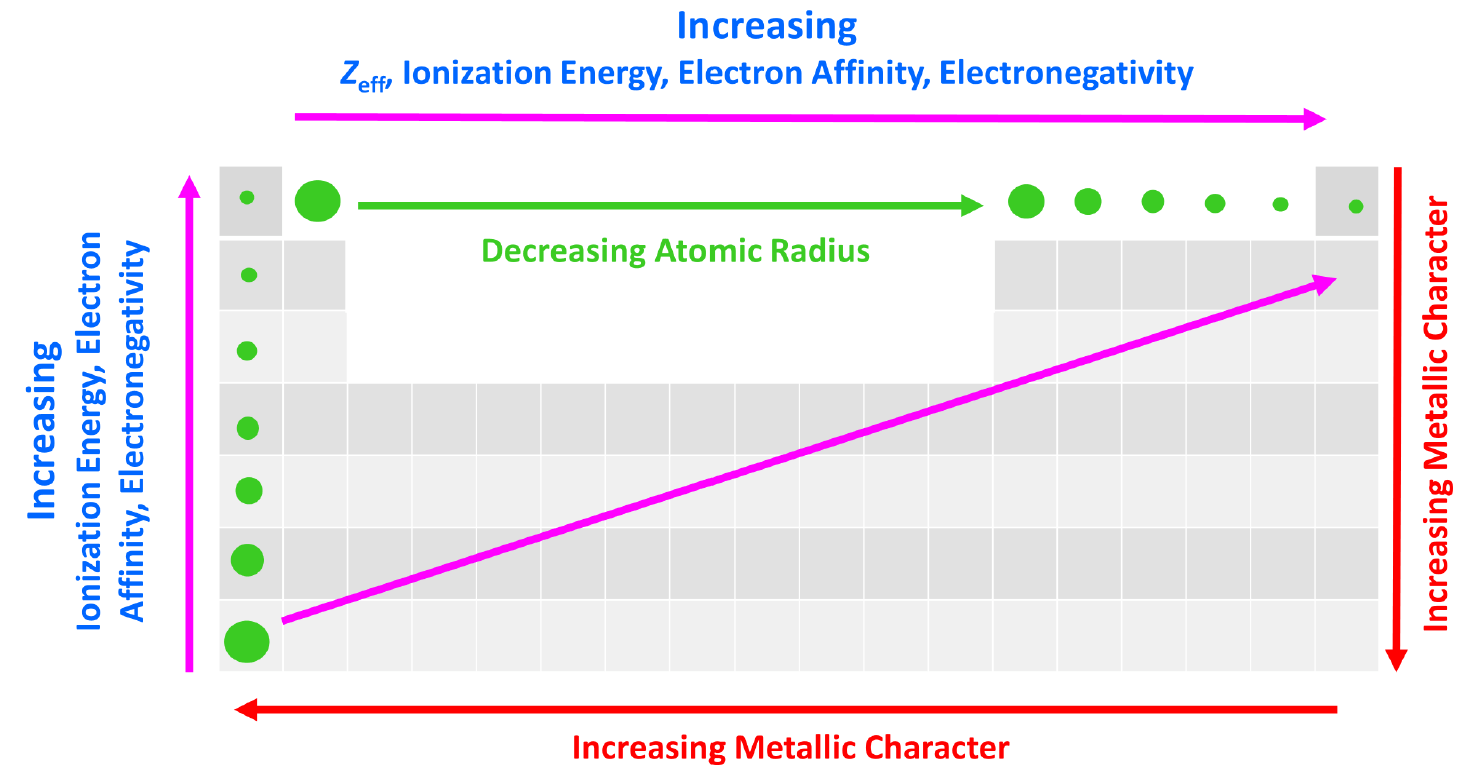
We can put together a general trend for the atomic radius, effective nuclear charge, ionization energy, electron affinity, and electronegativity.
You can look at these as how much an atom ‘likes’ electrons. The more we move to the right in the periodic table, the more the atoms like electrons, because of a greater nuclear charge which increases the ionization energy, electron affinity, and electronegativity. This results in smaller atoms because the nucleus holds the electrons closer and tighter.
This, in turn, means that the metallic character of the elements decreases as metals ‘don’t like’ electrons and tend to become cations.
Check this 95-question, Multiple-Choice Quiz on the Electronic Structure of Atoms including questions on properties of light such as wavelength, frequency, energy, quantum numbers, atomic orbitals, electron configurations, and more.
Check Also
- Atomic Orbitals
- Electron Configurations
- Electron Configurations of Ions
- Orbital Diagrams
- Aufbau’s Principle, Hund’s Rule, and Pauli’s Exclusion Principle
- Hund’s Rule
- Pauli Exclusion Principle
- Quantum Numbers (n, l, ml, ms)
- Bohr Model of the Hydrogen Atom
- Rydberg Formula
- The Photoelectric Effect
- Calculating The Energy of a Photon
- Effective Nuclear Charge
- Ionization Energy
- Electron Affinity
- Energy, Wavelength, and Frequency Practice Problems

