Valence electrons are those in the outermost principal energy level. For example, sodium has one valence electron, and chlorine has seven valence electrons: Na – 1s22s22p63s1, Cl – 1s22s22p63s23p5. The rest of the electrons in the lower energy levels are called core electrons.
The number of valence electrons, for main group elements, corresponds to their group number in the periodic table. For example, Mg is a Group 2A element and has two dots for two valence electrons, and C has four dots in the Lewis symbol since it is in Group 4A, and oxygen has six valence electrons:

This notation when the element is shown surrounded by its valence electrons is called Lewis dot symbols, and it is used for drawing the Lewis structure of molecules.
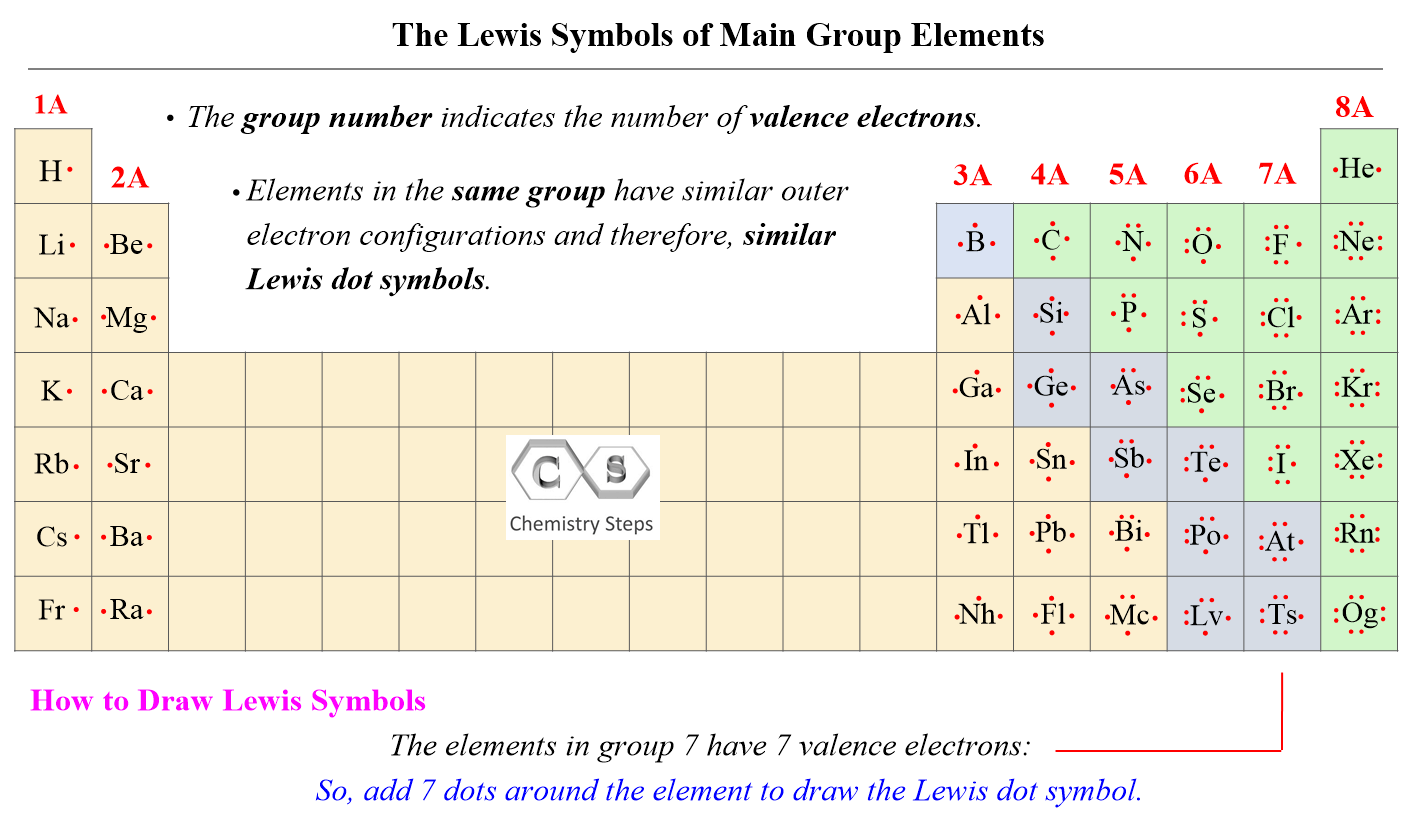
If it is noted as 14 instead of 4A, you can subtract 10 from the group number and determine the valence electrons.
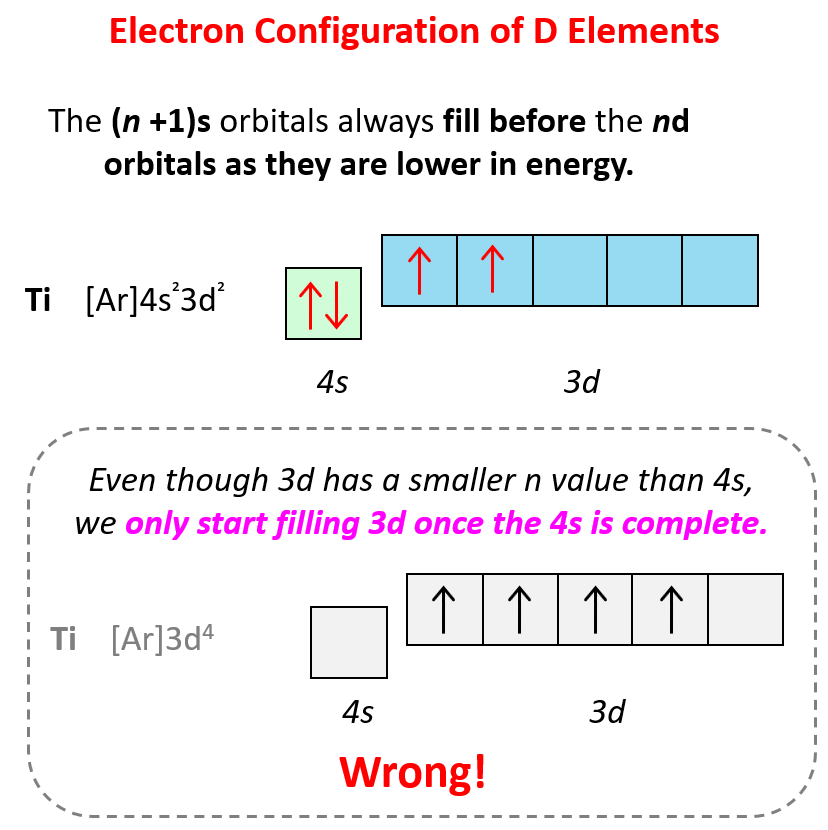
For d block elements, the outermost d electrons are also counted as valence electrons (ns + (n-1)d). For example, iron has eight valence electrons: Fe – 1s22s22p63s23p64s23d6, titanium has four electrons – Ti – 1s22s22p63s23p64s23d2.
Remember from the electron configurations that for transition metals, the (n+1)s energy level is filled before the (n)d level.
Because valence electrons are the farthest from the nucleus, they are the ones that are not bound as strongly and thus participate in chemical bonding and define the properties of the element.
Elements in the same group have similar chemical and physical properties. This is because they have the same valence electron configuration just different energy levels.
Without knowing the valence electrons, we cannot draw Lewis structures which means we cannot determine the geometry and hybridization of the atom.
Check Also
- Lewis Dot Symbols
- The Ionic Bond
- The Covalent Bond
- Sigma and Pi Bonds
- Electronegativity and Bond Polarity
- The Octet Rule
- Formal Charges
- Lewis Structures and the Octet Rule
- Lewis Structures Practice Problems
- Resonance Structures
- Chemical Bonding and Lewis Structures Quiz
