Sulfur is the central atom:
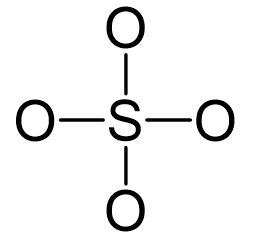
There are 4×6 + 6 + 2 = 32 electrons, and 8 of them are used to make 4 bonds. The four oxygens take 3 lone pairs each and no electrons are left:
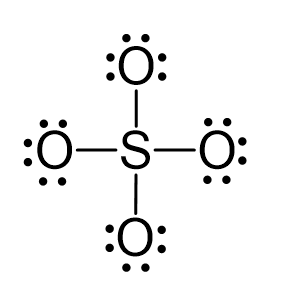
All the electrons have been used; however, two oxygens need to share a pair of electrons with the sulfur otherwise the ionic would be -4:
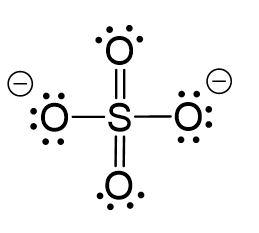
The central atom has 4 atoms and no lone pairs therefore, both geometries are tetrahedral.
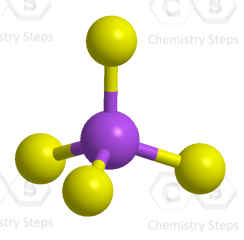
Steric number 4 corresponds to sp3-hybridization where the idealized bond angles are 109.5o.
Check Also
