In the previous post, we talked about the percent composition and its use for determining the empirical and molecular formulas of compounds. Remember, the percentage composition shows the amount of each element in a compound expressed as a mass percent and is calculated with the following formula:
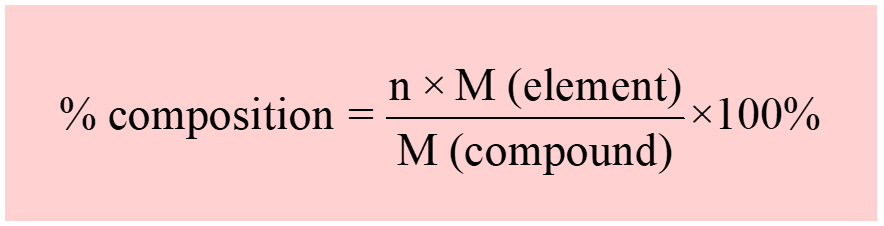
The empirical formula indicates the mole ratio of atoms in the simplest whole numbers. For example:
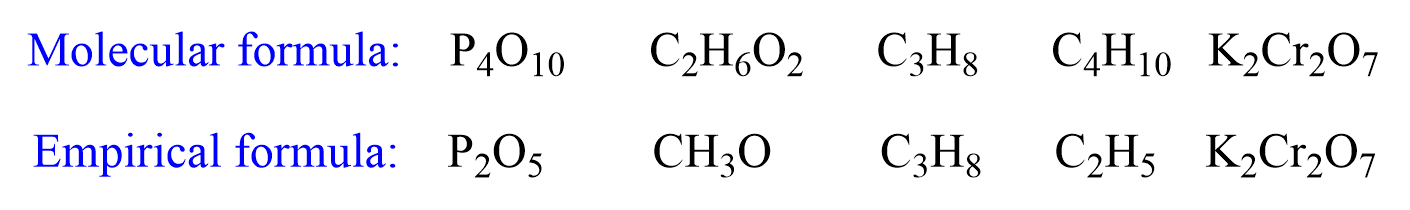
Notice that the empirical formula can be the same as the molecular formula if the numbers cannot be simplified any further.
Below is the summary of steps you need to determine the empirical and molecular formulas of the compound:

Use these principles to work on the practice problems on percent composition and empirical and molecular formulas.
Check Also
- The Mole and Molar Mass
- Molar Calculations
- Percent Composition and Empirical Formula
- Stoichiometry of Chemical Reactions
- Limiting Reactant
- Reaction/Percent Yield
- Stoichiometry Practice Problems
Practice
Calculate the percentage composition of butane, C4H10. How many percent of carbon and hydrogen, by mass, does butane contain?
What is the percent composition of lithium in Li3PO4 ?
What is the percent composition of water in Cupric sulfate pentahydrate, CuSO4·5H2O ?
How many grams of phosphorous are there in a 49.7 g sample of P2O5?
Determine the empirical formula for a compound that contains 18.75 % C, 6.25% H, and 75% O by mass.
Fructone is used in different types of perfumes as a synthetic fruit-aroma additive. It contains 55.17 % C, 8.046 % H and the rest being oxygen. Determine the empirical and molecular formula of Fructone considering that its molar mass is 174.0 g/mol.
What is the mass percent of the carbon in ethanol, C2H5OH?
Calculate the percent composition by mass of the steroid hormone cortisol with the molecular formula of C21H30O5.
Determine the molecular formula of each compound given the empirical formula.
(a) Empirical formula CH2 (M = 84 g/mol)
(b) Empirical formula NH2 (M = 80 g/mol)
(c) Empirical formula CHS (M = 180.4 g/mol)
Determine the empirical formula of the compound that is formed when 0.251 mol of nitrogen atoms are combined with 0.6273 mol of oxygen atoms.
In a reaction, 0.61764 g of carbon was combined with 7.309 g of chlorine. What is the empirical formula of the resulting product?
Determine the molecular formula of a compound that contains 34.2% C, 11.43% O, 22.0% P, 7.17% H, and 25.2% Cl, and has a molar mass of 140.55 g/mol.
Nicotine contains 74.0% carbon, 8.7% hydrogen, and 17.3% nitrogen by mass. What are the empirical and molecular formulas of nicotine considering its molar mass is 162.1 g/mol.
Cysteine is an important non-essential amino acid that contains 29.74 % carbon, 5.82 % hydrogen, 26.41 % oxygen,11.56 % nitrogen, and 26.47 % sulfur. What are the empirical and molecular formulas of cysteine considering it molar mass is 121.2 g/mol.
Muscle soreness during excessive physical activity is due to the synthesis of lactic acid that contains 40.0 % C, 6.71 % H, and 53.3 % O by mass. Determine the empirical and molecular formulas of lactic acid considering it molar mass is 90.08 g/mol.
Acetone is commonly used as a nail polish remover. When 0.2647 g of acetone was burned in a combustion apparatus, 0.6019 g of CO2 and 0.4925 g of H2O were formed. Determine the molecular formula of acetone if its molas mass is 58.08 g/mol.
Determine the empirical formula of a compound that contains 23.75% S, 23.75% O, and 52.5 % Cl by mass.
A) SOCl
B) SOCl2
C) S2OCl
D) SO2Cl2
E) ClSO4
Determine the empirical formula of a compound that contains 44.9% K, 18.4% S, and 36.7% O by mass.
A) KSO2
B) KSO3
C) KSO4
D) K2SO3
E) K2SO4
What is the empirical formula of a compound that contains 20.3 mg P and 69.7 mg Cl.
A) PCl
B) P2Cl3
C) PCl2
D) PCl3
E) P3Cl
What is the empirical formula of adipic acid if it contains 49.32% C, 43.84% O, and 6.85% H by mass?
A) C2HO3
B) C3H3O4
C) C3H5O2
D) C2H5O3
E) C3H3O
A 6.84-g sample of an iron oxide contains 4.78 g of metal. Determine the empirical formula for the compound.
A) FeO4
B) Fe2O
C) FeO2
D) FeO
E) Fe2O3
Determine the molecular formula of a compound that contains 22.75 % carbon, 67.15 % chlorine, and 10.10 % oxygen by mass, considering that its 0.045-mol sample weighs 7.133 g.
A) CClO
B) C3Cl3O
C) C2Cl2O2
D) C4Cl4O4
E) C5Cl5O5
Determine the formula of a compound if its 0.650 mol sample contains 3.914 × 1023 sodium atoms, 23.08 g of chlorine atoms, and 41.6 g of oxygen atoms.
A) NaClO3
B) NaClO5
C) NaClO
D) NaClO4
E) NaClO2
The elemental analysis of a 2.300-g sample of a compound containing only carbon, hydrogen, and oxygen produced 4.217 g of CO2 and 1.150 g of H2O. Determine the empirical formula of the compound.
A) C4H6O2
B) C3H4O2
C) C2H4O
D) C2H2O
E) CH2O3
The combustion analysis of a 31.9 g sample of a compound containing C, H, and O produced 72.5 g of CO2 and 29.69 g of H2O. Determine the empirical formula for the compound.
A) C3H6O
B) C3H6O2
C) CH3O
D) C2H4O
E) C3H4O2
Calculate the mass of oxygen in a 2.064 g sample of a compound containing only carbon, hydrogen, and oxygen if burning it in an excess of dioxygen produces 4.224 g CO2 and 1.296 g H2O.
A) 535 g
B) 384 g
C) 768 g
D) 512 g
E) 152 g
