Nitrogen is the central atom:
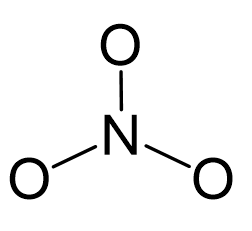
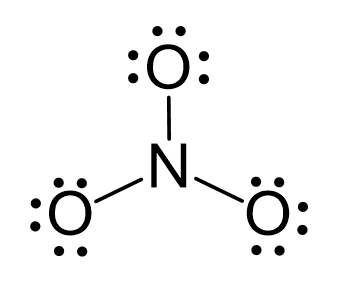
There are 5 + 3×6 + 1 = 24 electrons, and 6 are used to make the two covalent bonds. The oxygens get 6 electrons as three lone pairs:
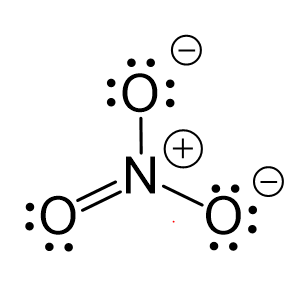
One lone pair from an oxygen is used to make a π bond with the nitrogen and thus making the ionic charge -1:
The central atom has 3 atoms and no lone pairs; therefore, both geometries are trigonal planar.
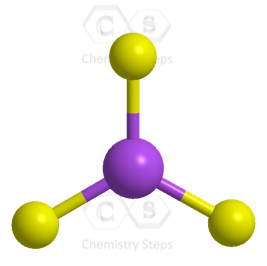
The steric number (the sum of the number of the atoms and lone pairs) of the carbon is 3 which corresponds to sp2-hybridization.
Check Also
