In the previous post, we saw that the ionic bond is an electrostatic interaction between ions that are formed when one electron(s) is transferred from one atom to the other.
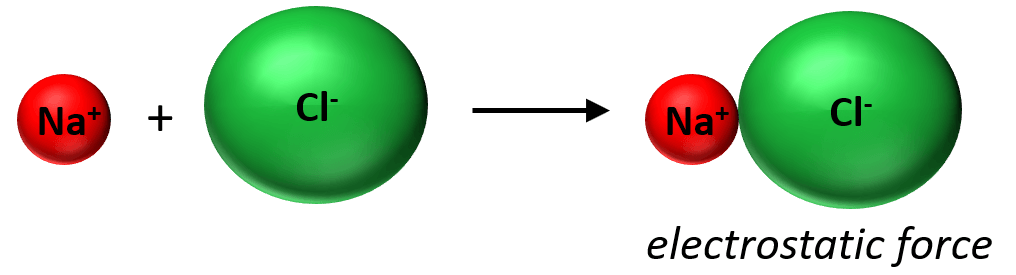
The reason for this electron transfer is the large difference in the electron affinities that the elements have. In contrast, when the elements have a nearly equal electron affinity, they tend to form a bond by sharing two electrons. Each atom contributes one electron to making this bond which we call a covalent bond. The covalent bond can be shown with Lewis dot symbols. Now the covalent bond is not entirely different than the ionic bond in that there are still electrostatic interactions between the bonding electrons and the nuclei.
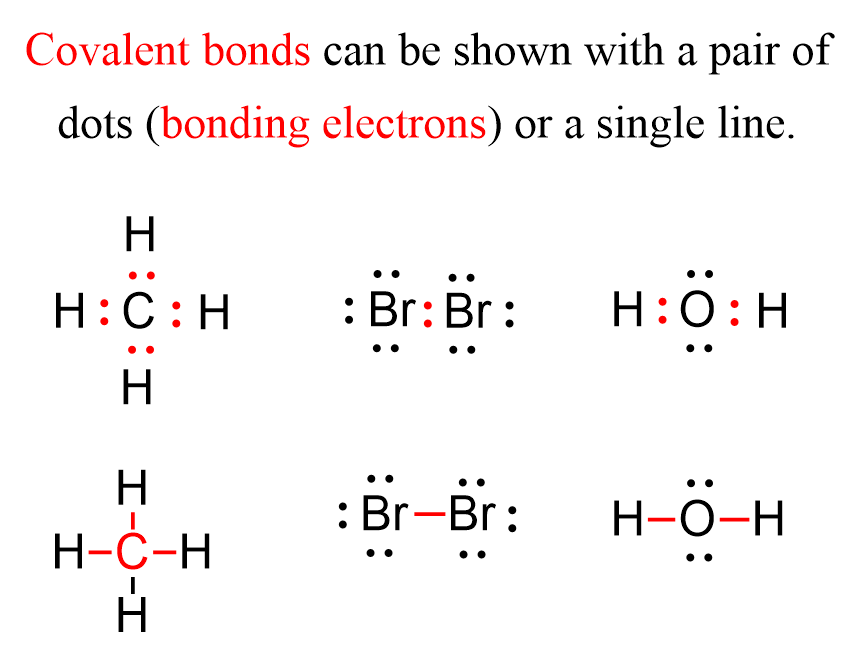
Notice that for simplicity, the bonding electrons, meaning the covalent bonds, are sometimes shown as a single line.
The shared electrons are called bonding electrons or a bonding (electron) pair. The other electrons on any of the atoms that are not part of a covalent bond are called nonbonding electrons are lone pairs of electrons (lone pairs). For example, in water there are two nonbonding electrons on the oxygen:

All of these structures are called Lewis structures since we show the electrons are Lewis dot symbols. A Lewis structure is a representation of molecules where the covalent bonds are shown either as lines or as pairs of dots placed between connected atoms. The lone pairs, on the other hand, are shown as pairs of dots on individual atoms. We will discuss Lewis structures and the ways of drawing them in the next article.
The Octet Rule
We can find a pattern in the Lewis structures where some of the atoms are surrounded by eight electrons:
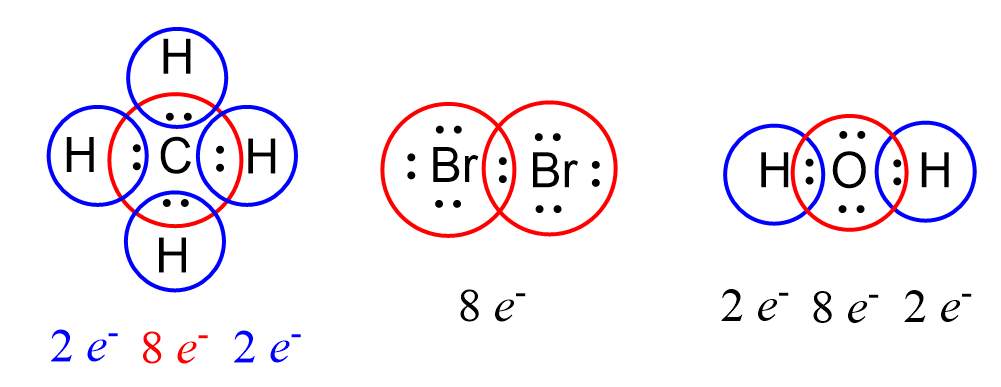
This is an illustration of the octet rule which states that atoms tend to form bonds until they are surrounded by eight valence electrons. The pattern of having eight valence electrons is characteristic of noble gases which all have a valence shell configuration of ns2np6 except He – 1s2. Remember that noble gases are inert as they are very stable and the reason for this is the completely filled electron configuration having 2 electrons in the s, and 6 electrons in the p level.
It is important to mention that the octet rule does not apply to all the elements. Quite the opposite, while it may be applicable to many elements in certain molecules, strictly speaking, it is exclusive to the nonmetals in the second row of the periodic table. That is B, C, N, O, and Cl. These elements can never have more than eight electrons around them simply because they do not have available orbitals to accommodate electrons in the stable, bonding energy level.
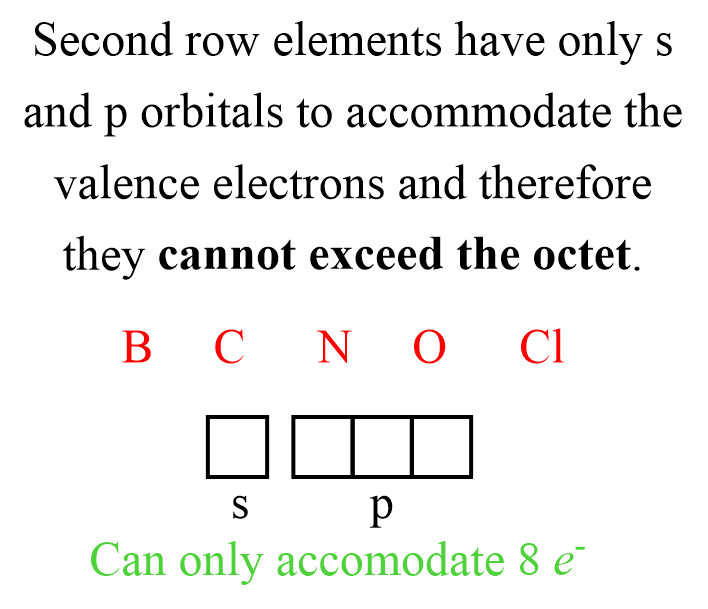
Elements from the third row and below can be surrounded by more than 8 electrons since they have the d orbitals available, and in this case we say that they exceed the octet.
For example, sulphur in SO3 has three double bonds which correspond to 12 electrons:
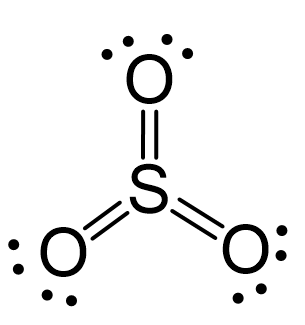
When atoms have more than or less than 8 electrons, they are often charged because of the imbalance between the positive and negative charges. These are called formal charges, and we will devote a separate post to discussing them.
The Covalent Bond and Orbitals
Remember, from the discussions about the atomic structure, that electrons are located in orbitals. We have also seen this when writing the electron configurations of elements (1s22s22p6 etc.) where we indicate what types of orbitals accommodate the given electrons.
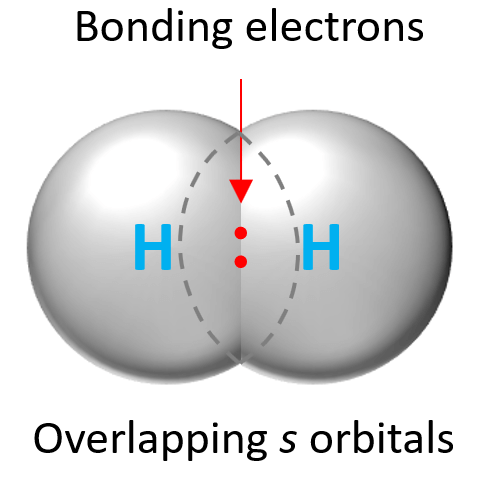
Now because the electrons are located in orbitals, in order to form a covalent bond, the orbitals of the atoms making the bond need to overlap. For example, the H2 molecule is formed by the overlap of two s orbitals:
Double and Triple Covalent Bonds
So far, we have seen how two atoms share a pair of bonding electrons which corresponds to a single bond. Now, when two pairs of electrons are shared, then a double bond is formed between the atoms. For example, in carbon dioxide, CO2, the carbon is connected with a double bond to each oxygen on the side:
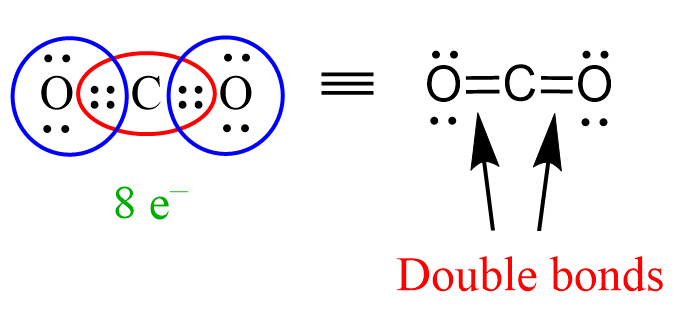
Notice that the carbon and both oxygens have an octet because we count the additional bonding pair toward the octet of each atom.
Similar to this, when three pairs of bonding electrons are shared, a triple bond is formed between the atoms. A good example showing this is the structure of N2:
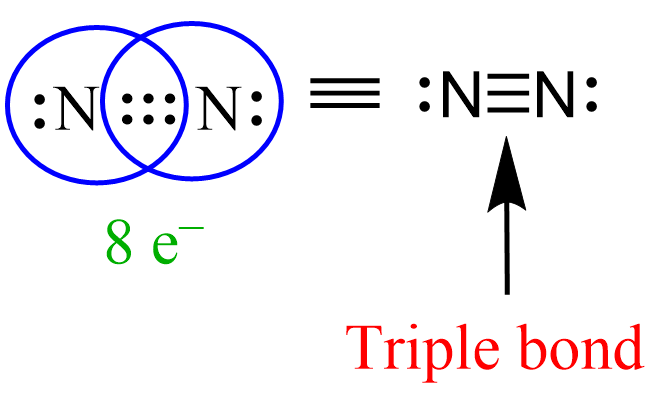
There are 6 electrons shared and a lone pair on each nitrogen which satisfies the octet rule for both of them.
In general, double and triple bonds are shorter and therefore, stronger than single bonds.
Check Also
- Lewis Dot Symbols
- The Ionic Bond
- Sigma and Pi Bonds
- Electronegativity and Bond Polarity
- The Octet Rule
- Formal Charges
- Lewis Structures and the Octet Rule
- Lewis Structures Practice Problems
- Resonance Structures
- The VSEPR Model
- VSEPR Theory Practice Problems
- Hybridization of Atomic Orbitals
- sp, sp2, sp3, sp3d, and sp3d2Hybridization Practice Problems
Check this 90-question, Multiple-Choice Quiz on Chemical Bonding:

