Colligative (collective) properties are those that depend only on the number of particles dissolved in solution, and not on the type of particle. For example, salt is sometimes scattered on icy roads to melt the ice.

What happens here is the saltwater solution freezes at lower temperatures than pure water does. As a result, the road may not get icy even below 0 °C, and the extent to which the freezing point is lowered depends on the amount of the salt. Importantly, it does not have to be the able salt, NaCl. The effect would be identical if, for example, KCl was added. Being more affordable and not hazardous is what makes NaCl a preferred candidate over other salts.
There are four colligative properties: vapor pressure lowering, boiling point elevation (Tb), freezing point depression (Tf), and osmotic pressure (ᴨ), and this is how, specifically, the properties of a pure solvent compare with those of a solution:
Colligative Properties
How the properties of solutions change compared to those of a pure solvent:
- The vapor pressure of the solution is lower.
- The boiling point of the solution is higher.
- The freezing (or melting) point of the solution is lower.
- The solution causes osmosis, the flow of solvent from a solution of lower solute
concentration to one of higher solute concentration a semipermeable membrane.
The key equations for quantitative description of the colligative properties are summarized in the chart below:
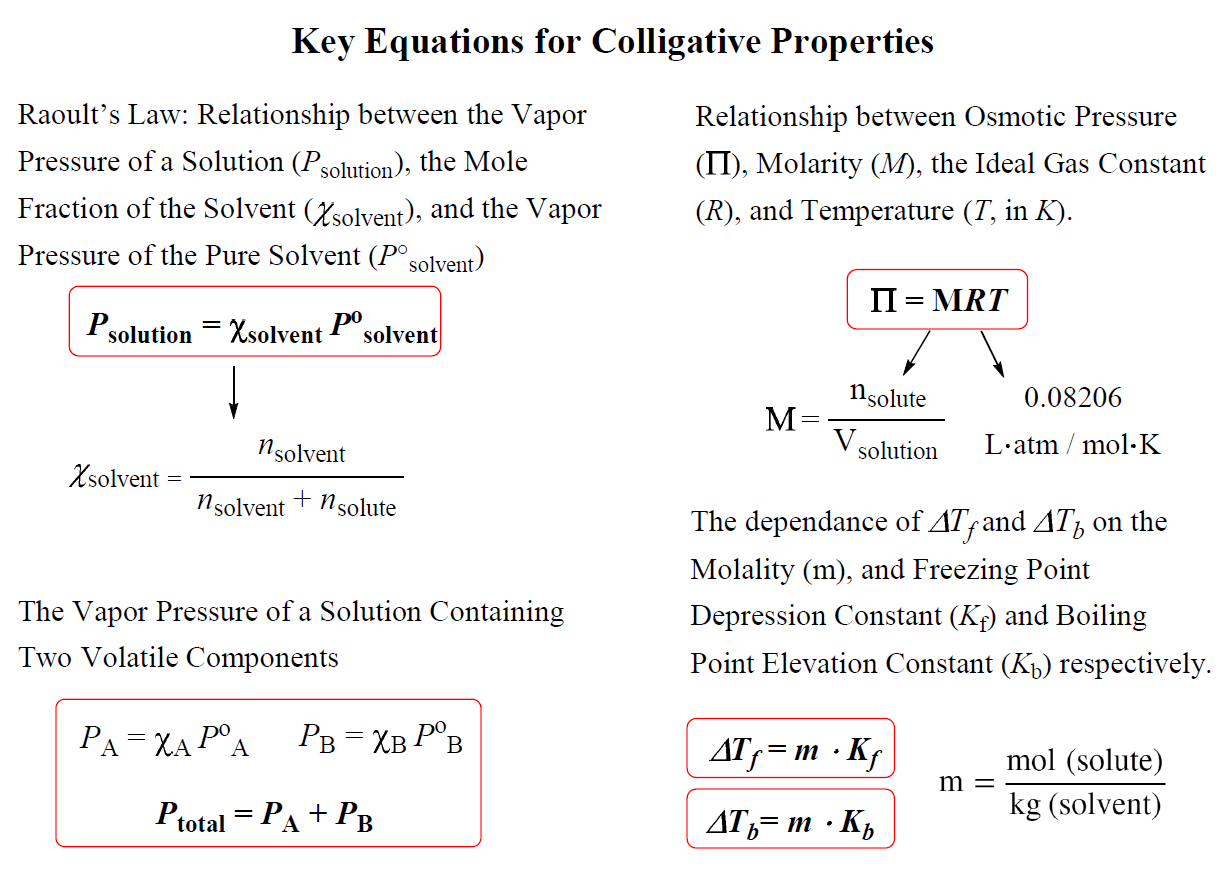
In the next few articles, we will go over each colligative property in more detail.
Check Also
