First, we need to draw the Lewis structure of CO.
In short, these are the steps you need to follow for drawing a Lewis structure:
1. Write the correct skeletal structure for the molecule.
* Hydrogen atoms are always terminal (only one bond)
* Put more electronegative elements in terminal positions
2. Sum the valence electrons from all the atoms.
3. Use a pair of electrons to form a bond between each pair of bound atoms.
4. Add the remaining electrons to satisfy the octet for a more electronegative atom first.
5. If any atoms lack an octet, make a double or triple bond to give them an octet.
There are 4 + 6 = 10 valence electrons, so let’s use two of these to make a C-O bond:
![]()
There are eight electrons left, and we give 6 to oxygen as the more electronegative atom. This gives the oxygen an octet, and the other two electrons can now go to the carbon:
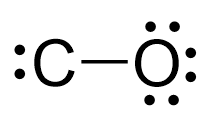
At this point, we need to make sure that carbon also has an octet, and for this two of the oxygen’s lone pairs need to be shared with it to make two additional bonds.
![]()
Remember these are pi bonds, because multiple bonds are composed of one sigma bond, and the others are pi bonds.
Let’s also consider the formal charges because, as you may have noticed, neither the carbon nor the oxygen follow their standard bonding pattern:

FC= V – (N + B)
Where:
V – number of valence electrons
N – number of nonbonding electrons
B – number of bonds
So, the formal charge of the oxygen will be
FC (O) = 6 – (2 + 3) = +1
For the carbon, the formal charge is
FC (C) = 4 – (2 + 3) = -1
So, overall, the molecule is neutral, however, two atoms have formal charges that balance each other.
Let’s also discuss the hybridization of the atoms in CO. Remember, the shortcut to determining the hybridization is the steric number (electron domain).

If the steric number is 4, it is sp3
If the steric number is 3 – sp2
If the steric number is 2 – sp
Both atoms have a steric number of 2 (one atom and one lone pair), and therefore, their hybridization is sp.
For each atom, the connected atom and the lone pair are in sp orbitals at 180o, and the other two 2p orbitals are used for making the pi bonds:
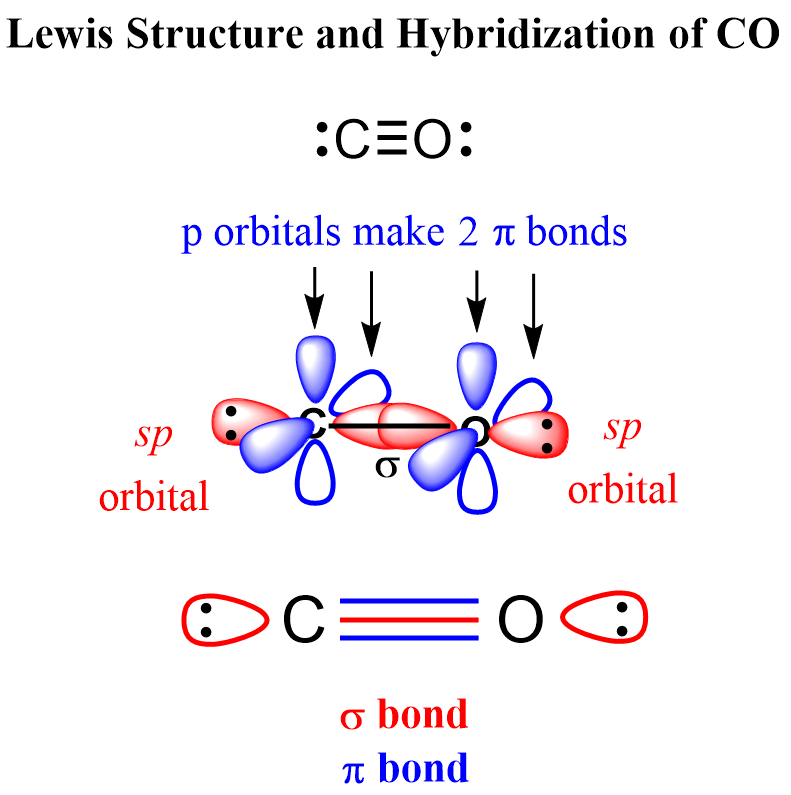
Both electron and molecular geometries are linear because it is a diatomic molecule and each atom has only one lone pair which makes the steric number one.
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Check Also
- The VSEPR Model
- VSEPR Theory Practice Problems
- Hybridization of Atomic Orbitals
- sp, sp2, sp3, sp3d, and sp3d2 Hybridization Practice Problems

