To determine if CH2O (formaldehyde ) is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here.
The carbon is the central atom:
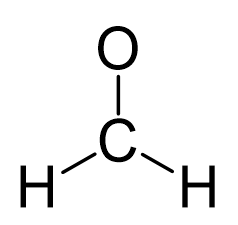
There is a total of 4 + 2×1 + 6 = 12 electrons, and 6 are already used for making the bond. The remaining 6 go to oxygen as it is the most electronegative atom.
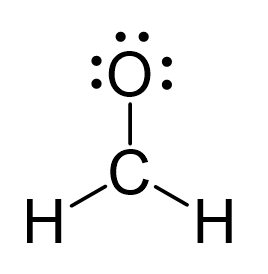
Because the carbon lacks an octet, we use one lone pair from the oxygen to make a π bond with the carbon, and thus, a double bond is formed.
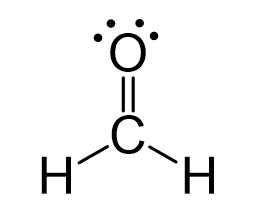
The central atom has three bonds and no lone pairs, therefore, both the electron and molecular geometries are trigonal planar.
In general, remember that oxygens on terminal positions are always going to have 2 bonds and 2 lone pairs of electrons unless there is a formal charge on them.
Knowing these patterns for bonding, lone pairs, and formal charges will make these types of exercises a lot easier.

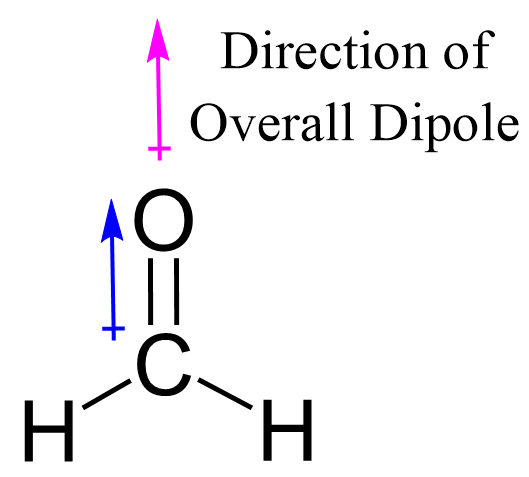
Now, the polarity: The first thing here is to determine if the C-O bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar.

- If the difference in electronegativity is less than 0.5, the electrons are about equally shared between the two atoms, forming a nonpolar a covalent bond.
- If the difference in electronegativity is between 0.5 and 1.7, we have a polar covalent bond.
- A difference of 1.7 or higher is so large that the electrons are no longer shared, and an ionic bond is formed. Ionic bonds are formed between metals and nonmetals.
There is one polar bond in CH2O which is the C=O bond because the difference in electronegativity values is E (O) – E(C) = 3.5 – 2.5 = 1.0 while for the C-H bond, it is only 0.4. The electron and molecular geometry of CH2O are trigonal planar and the molecule is polar as there are no other dipoles than the one of the C=O bond.
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Check Also

