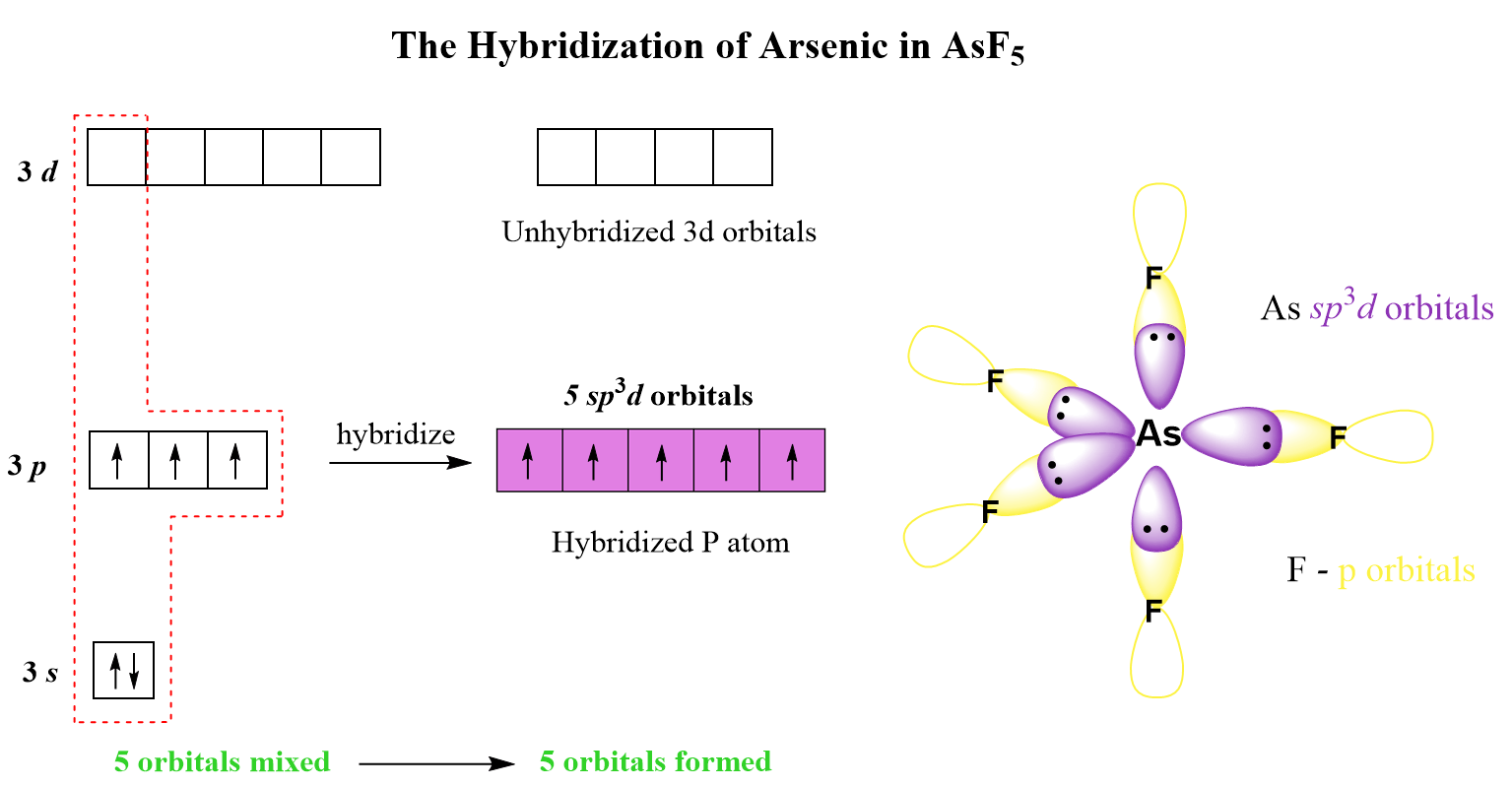
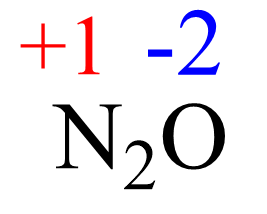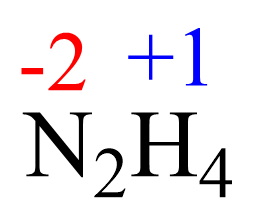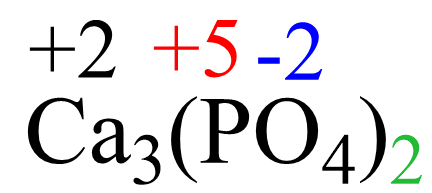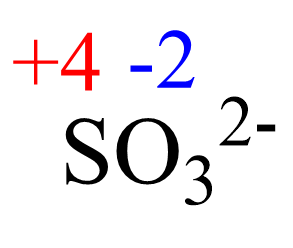AsF5 Lewis Structure, Geometry, and Hybridization
In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of AsF5. AsF5 Lewis Structure The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons … Read more