To determine if BeCl2 is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here.
Be is the central atom and there are a total of 2 + 2×7 = 16 valence electrons, and we use four of them to make the bonds.
![]()
The remaining 12 go on the Cl atoms as lone pairs:
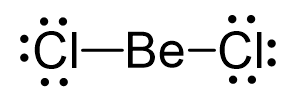
The steric number is 2, and there are no lone pairs on the central atom. Therefore, both geometries are linear.
Now, the polarity: The first thing here is to determine if the Be-Cl bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar.

- If the difference in electronegativity is less than 0.5, the electrons are about equally shared between the two atoms, forming a nonpolar a covalent bond.
- If the difference in electronegativity is between 0.5 and 1.7, we have a polar covalent bond.
- A difference of 1.7 or higher is so large that the electrons are no longer shared, and an ionic bond is formed. Ionic bonds are formed between metals and nonmetals.
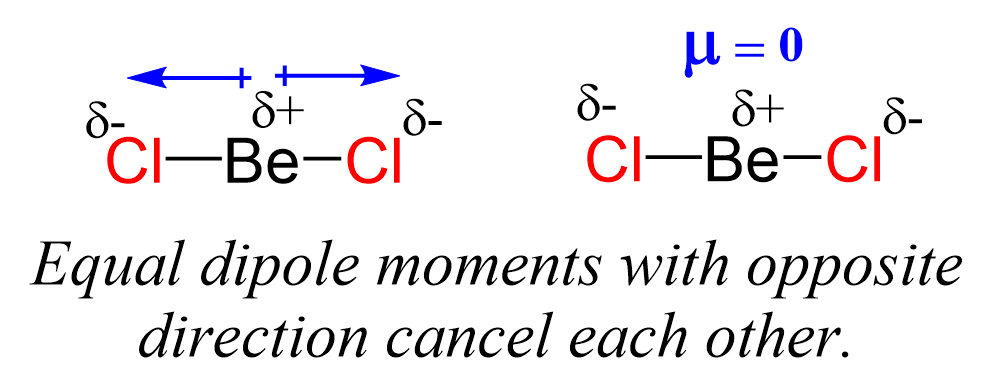
Even though the Be-Cl bond is polar, the symmetrical shape of the molecule results in no molecular dipole, and therefore, it is nonpolar.
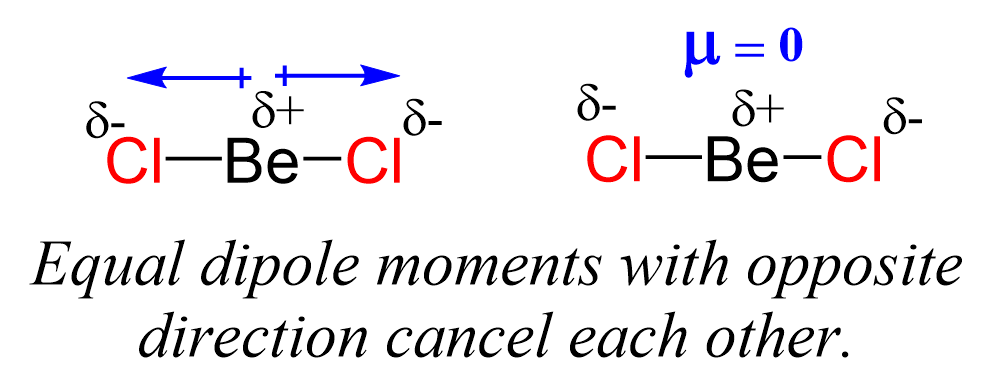
Remember, the net dipole of the molecule is the vector sum of all the dipoles and here it equals zero because the bonds are equivalent and pointing in opposite directions.
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Check Also
- The VSEPR Model
- VSEPR Theory Practice Problems
- Hybridization of Atomic Orbitals
- sp, sp2, sp3, sp3d, and sp3d2 Hybridization Practice Problems