In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of AsF5.
AsF5 Lewis Structure
The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na – 1s22s22p63s1, Cl – 1s22s22p63s23p5
The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:
For d block elements, the outermost d electrons are also counted as valence electrons (ns + (n-1)d). For example, iron has eight valence electrons: Fe – 1s22s22p63s23p64s23d6.
Arsenic is the central atom:
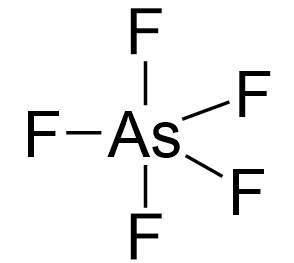
Arsenic is in group 5, so it has five valence electrons, while chlorine, being in group 7, has seven valence electrons. Therefore, there are, in total, 5×7 + 5 = 40 electrons, out of which, 10 are used to make 5 covalent bonds. All the remaining 30 are divided between the five fluorine atoms, each taking 6 electrons as 3 lone pairs:
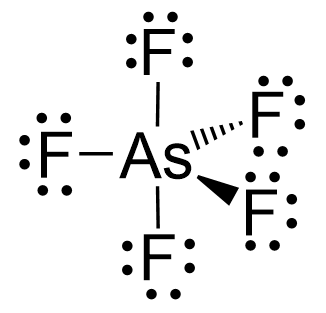
There are 5 atoms and no lone pairs on the central atom, therefore, both geometries are trigonal bipyramidal:

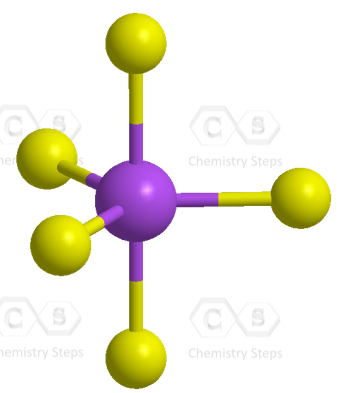
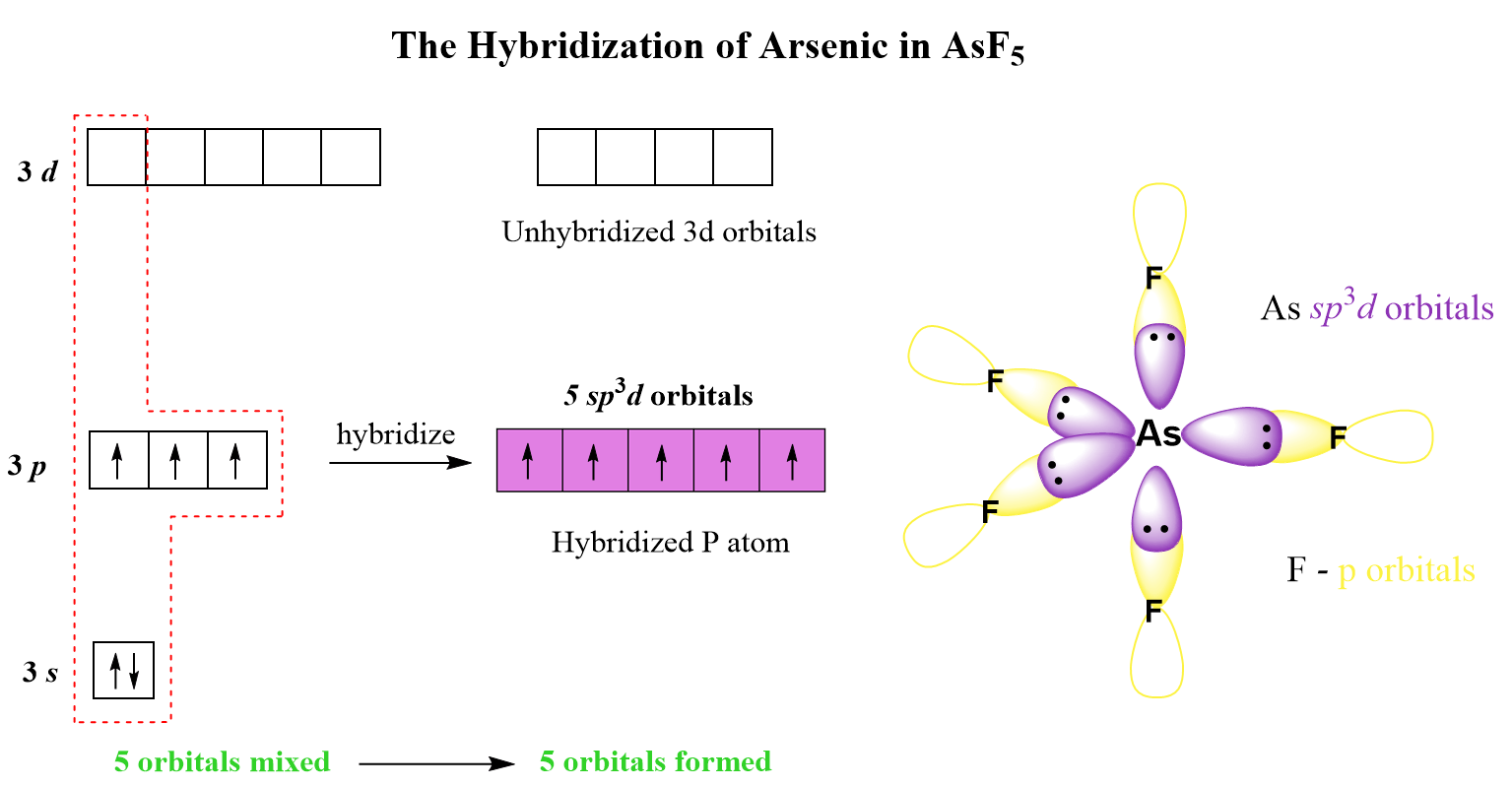
AsF5 Hybridization
The steric number of arsenic is 5 – five atoms, with no lone pairs, and therefore, the hybridization is sp3d.
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Check Also

