To determine if BrF3 is polar or nonpolar, we need to first draw the Lewis structure and determine its geometry.
The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na – 1s22s22p63s1, Cl – 1s22s22p63s23p5
The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:
For d block elements, the outermost d electrons are also counted as valence electrons (ns + (n-1)d). For example, iron has eight valence electrons: Fe – 1s22s22p63s23p64s23d6.
So, oxygen is in group 6A, and therefore, it has 6 valence electrons, thus O3 has 3 x 6 = 18 valence electrons.
Next, we need to connect the atoms in the correct order and add the electrons as bonds and lone pairs.
In short, these are the steps you need to follow for drawing a Lewis structure:
1. Write the correct skeletal structure for the molecule.
* Hydrogen atoms are always terminal (only one bond)
* Put more electronegative elements in terminal positions
2. Sum the valence electrons from all the atoms.
3. Use a pair of electrons to form a bond between each pair of bound atoms.
4. Add the remaining electrons to satisfy the octet for a more electronegative atom first.
5. If any atoms lack an octet, make a double or triple bond to give them an octet.
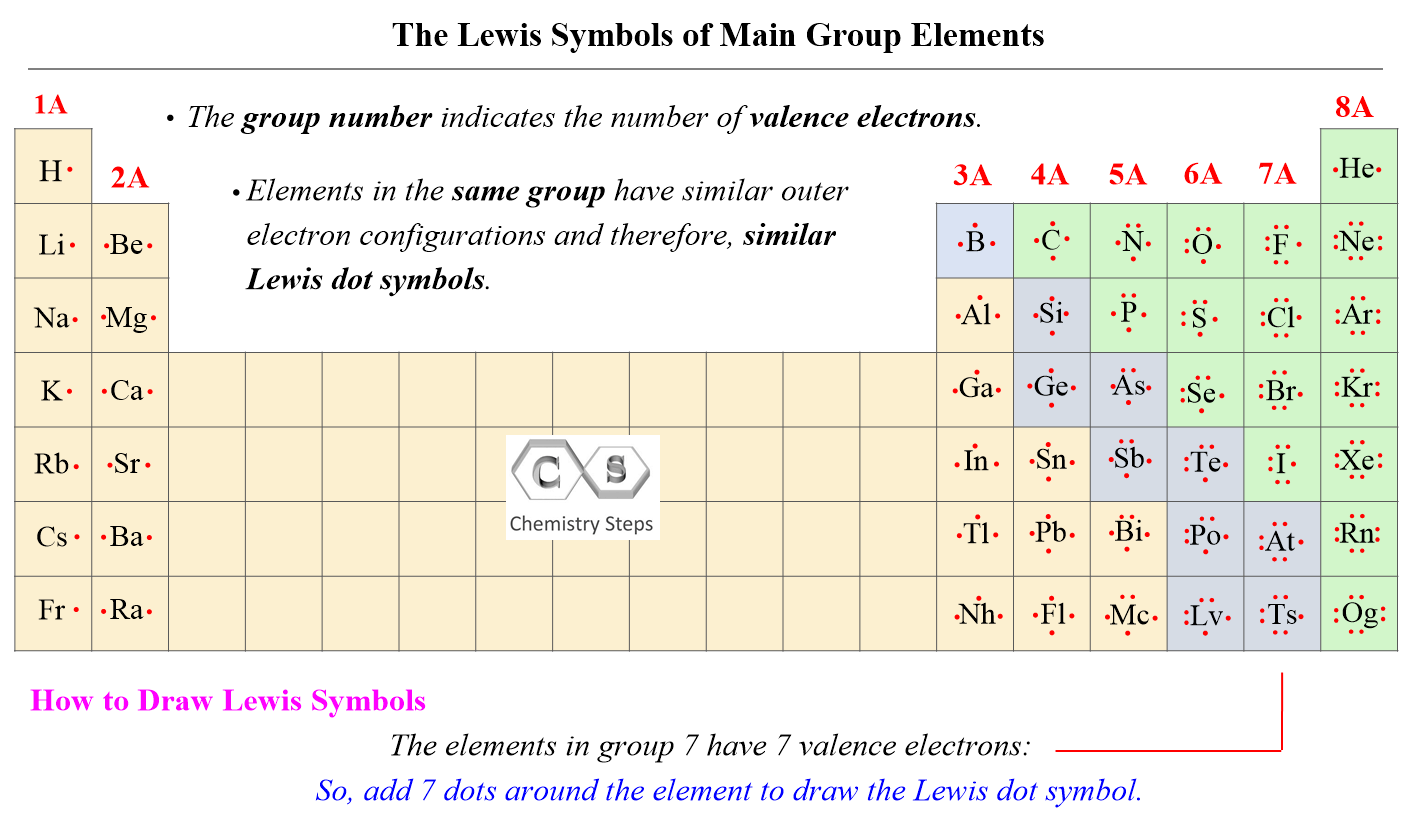
Br is the central atom:
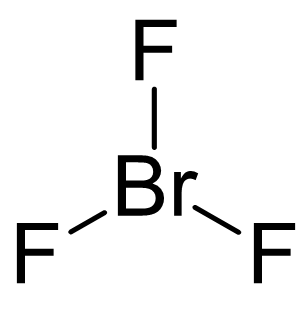
There are 7 + 3×7 = 28 electrons and 6 are taken to make three covalent bonds. Each fluorine takes 6 electrons, therefore there are 28 – (6 + 3×6) = 4 electrons left, which go on the Br as two lone pairs:
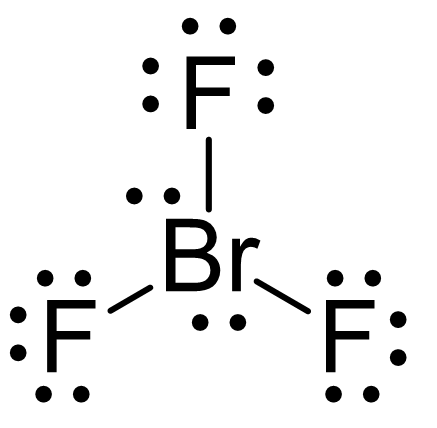
The central atom has 3 atoms and 2 lone pairs, therefore, the electron geometry is trigonal bipyramidal, while the molecular geometry is T-shaped:
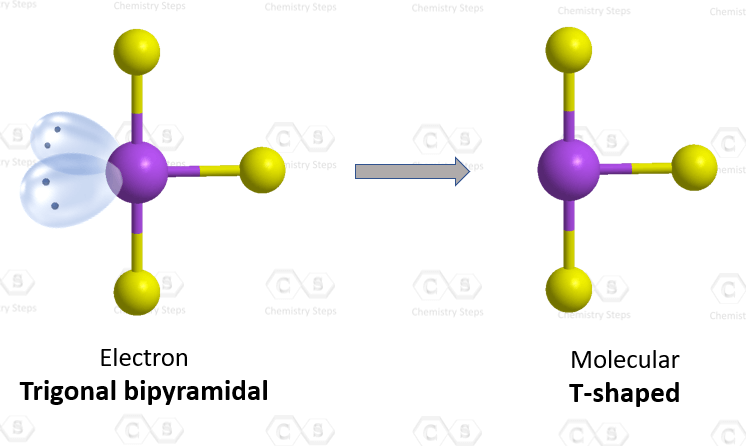
Notice that the lone pair does not go in axial positions (up or down).
Now, the polarity: The first thing here is to determine if the Br-F bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar.

- If the difference in electronegativity is less than 0.5, the electrons are about equally shared between the two atoms, forming a nonpolar a covalent bond.
- If the difference in electronegativity is between 0.5 and 1.7, we have a polar covalent bond.
- A difference of 1.7 or higher is so large that the electrons are no longer shared, and an ionic bond is formed. Ionic bonds are formed between metals and nonmetals.
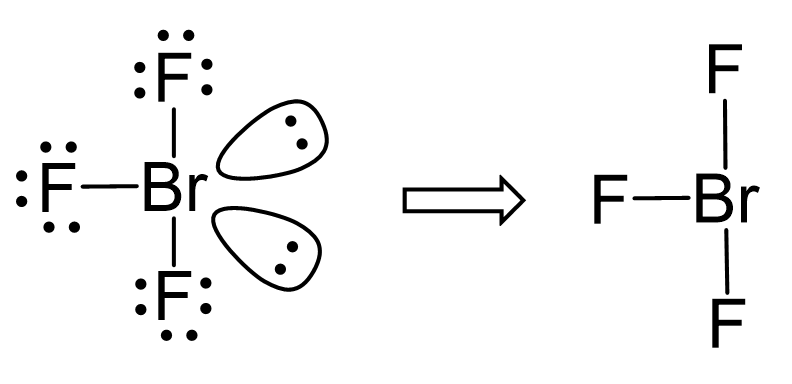
The difference in electronegativities is 1.2, and therefore, Br-F is a polar covalent bond. We have three dipoles in the molecule, so how do we determine if it is polar or not?
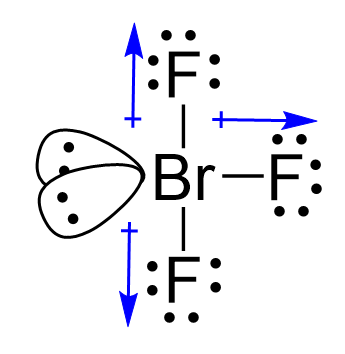
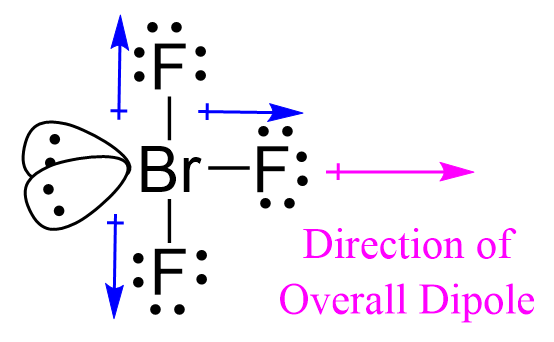
The molecule is polar if it has a dipole moment, and the molecular dipole is the vector sum of all the dipoles.
Two of the Br-F bonds balance each other because they are at 180o, but the third
one, pointing to the right here, is not balanced, and therefore, the molecular polarity points toward the fluorine on the right as drawn:
Check this 99-question multiple-choice quiz on Geometry and Hybridization:
Check Also
- The VSEPR Model
- VSEPR Theory Practice Problems
- Hybridization of Atomic Orbitals
- sp, sp2, sp3, sp3d, and sp3d2 Hybridization Practice Problems

