Br is the central atom, so we can draw a preliminary skeletal structure:
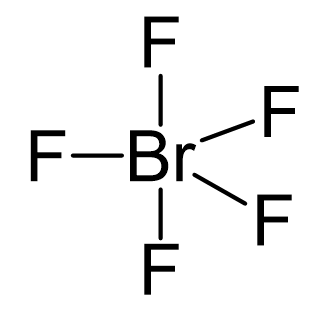
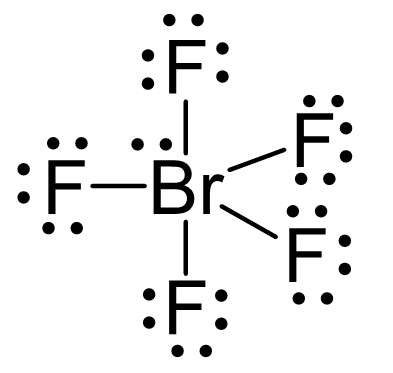
There are 5×7 + 7 = 42 electrons, out of which, 10 are used to make 5 covalent bonds. The remaining 30 are divided between the five fluorine atoms, each taking 6 electrons as 3 lone pairs, and Br takes the last pair of electrons:
There are 5 atoms and one lone pair on the central atom, therefore, the electron geometry is octahedral, while the molecular geometry is square pyramidal:
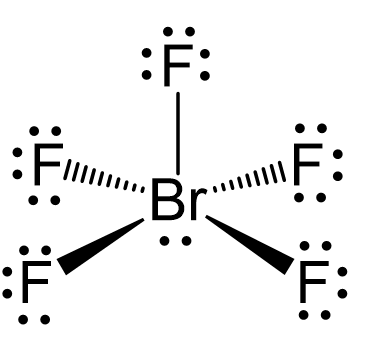
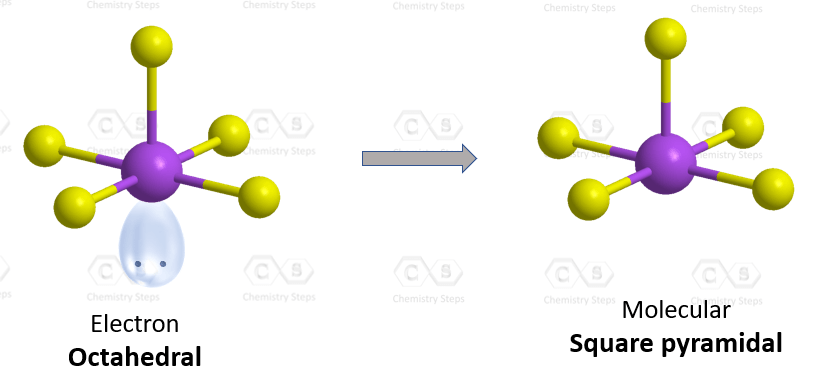
There are 6 units (atoms and lone pairs) on the central atom, and to accommodate them, it needs 6 orbitals which is achieved through sp3d2 hybridization.
Check Also
