In these practice problems, we will work on determining the electron and molecular geometry of molecules and ions using the VSEPR theory.
Practice
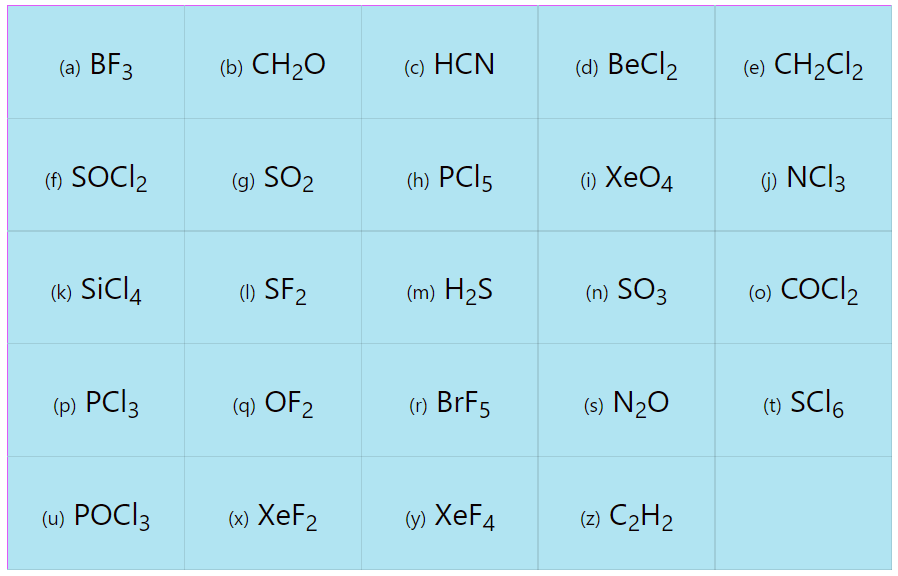
Determine the electron geometry and molecular geometry of the following molecules using the VSEPR model.
| (a) BF3 | (b) CH2O | (c) HCN | (d) BeCl2 | (e) CH2Cl2 |
| (f) SOCl2 | (g) SO2 | (h) PCl5 | (i) XeO4 | (j) NCl3 |
| (k) SiCl4 | (l) SF2 | (m) H2S | (n) SO3 | (o) COCl2 |
| (p) PCl3 | (q) OF2 | (r) BrF5 | (s) N2O | (t) SF6 |
| (u) POCl3 | (x) XeF2 | (y) XeF4 | (z) C2H2 |
Determine the electron and molecular geometry of each ion using the VSEPR theory: (a) NH4+, (b) H3O+, (c) CN– (d) SCN–, (e) CO32-, (f) ClO3–, (g) SO42- (h) PO43- (i) SO32– (j) NO2– (k) BF4– (l) NO3–.
| (a) NH4+ | (b) H3O+ | (c) CN– | (d) SCN– | (e) CO32- |
| (f) ClO3– | (g) SO42- | (h) PO43- | (i) SO32– | (j) NO2– |
| (k) BF4– | (l) NO3– |
For each molecular geometry, determine if there are any lone pairs on the central atom and name the molecular and electron geometries accordingly:
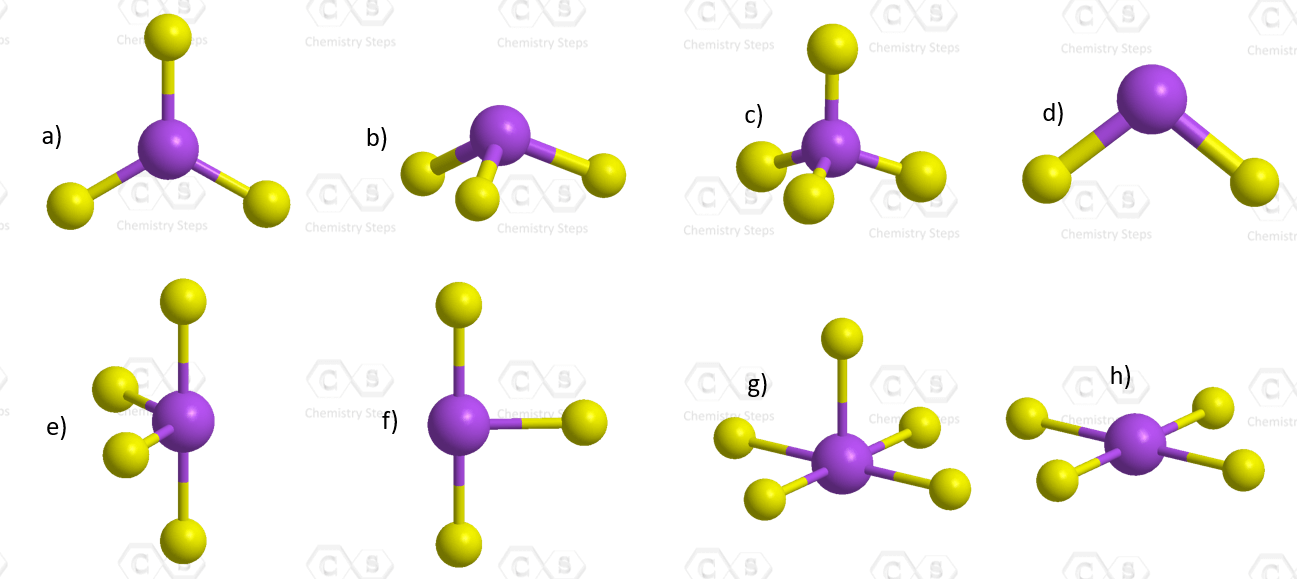
Below is shown the molecular geometry of ReF7. How would you name this geometry and what bond angles would you expect it to have?
For each marked atom, add any missing lone pairs of electrons to determine the steric number, electron and molecular geometry, approximate bond angles, and the hybridization state.
You can also download the questions as a PDF worksheet to print and work on here.
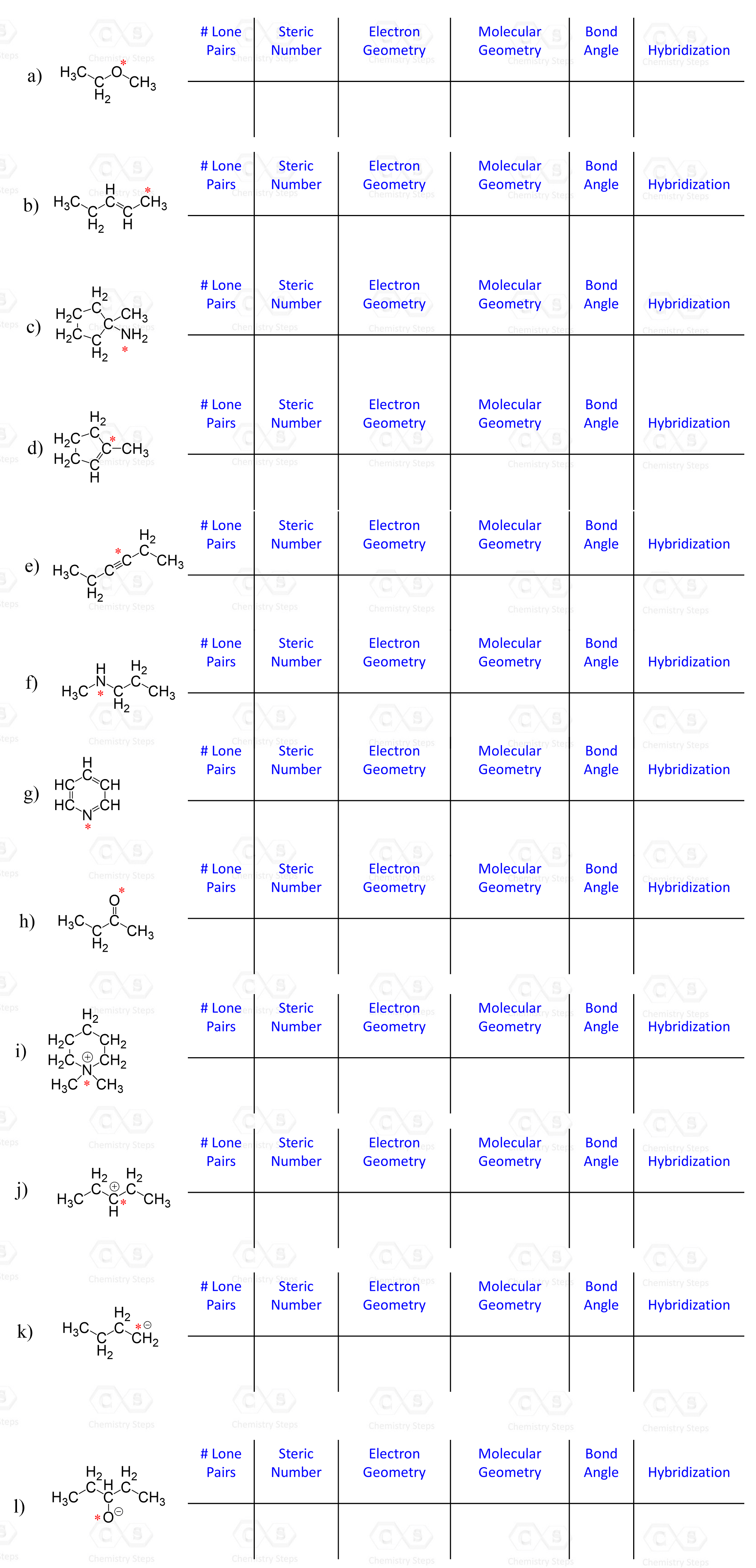
Determine the molecular geometry of each marked atom in the following molecule:

What is the molecular geometry of the nitrogen in the following molecules?

What is the molecular geometry of the nitrogen in the following molecules?

Determine the approximate bond angle in the following molecule:

Determine the approximate bond angle in the following molecule:

The angle between carbon 2 and the lone pair on the nitrogen is:

Check Also
- Lewis Dot Symbols
- The Ionic Bond
- The Covalent Bond
- Sigma and Pi Bonds
- Electronegativity and Bond Polarity
- The Octet Rule
- Formal Charges
- Lewis Structures and the Octet Rule
- Lewis Structures Practice Problems
- Resonance Structures
- The VSEPR Model
- Hybridization of Atomic Orbitals
- sp, sp2, sp3, sp3d, and sp3d2Hybridization Practice Problems